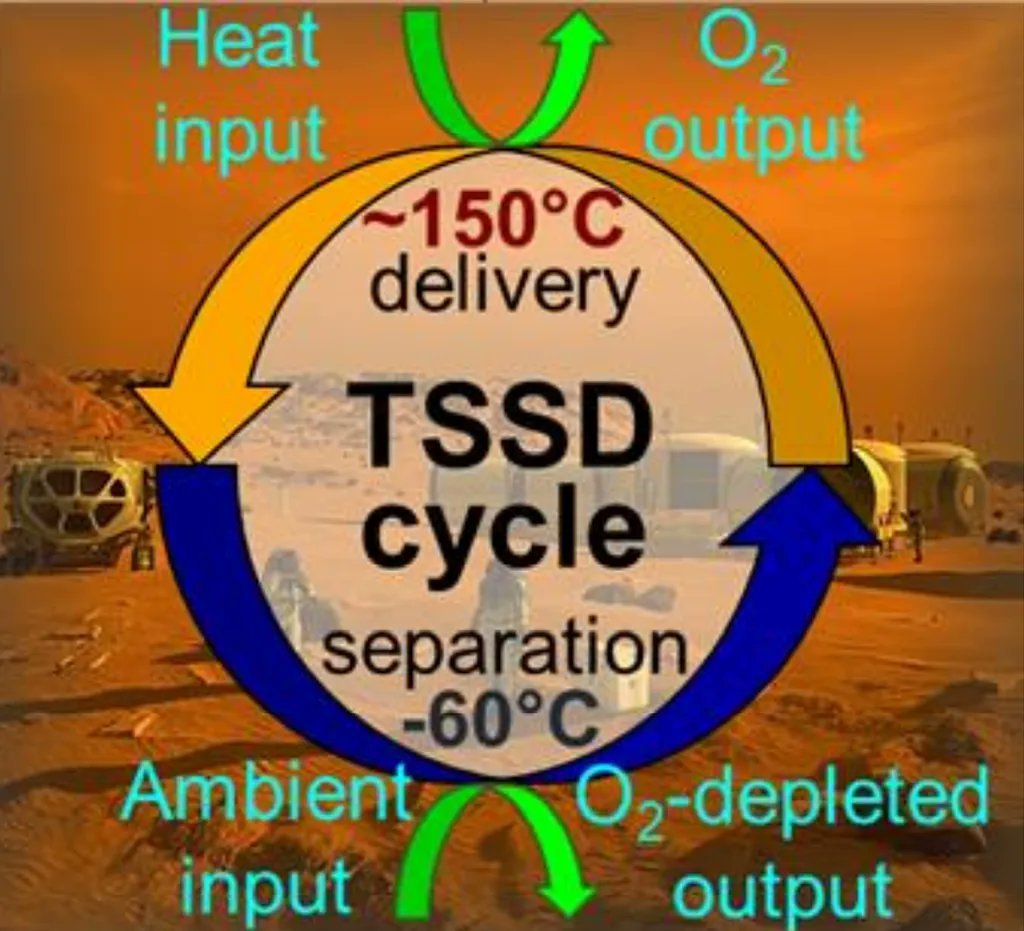
Viking’s biochemistry experiments have been among the most hotly debated scientific results of all time. The lander famously collected samples from the Red Planet in 1976, in an experiment called “Label Release.” Scientists watched with bated breath as oxygen was released from the sample after it was subjected to a liquid slurry. They were then left scratching their heads as that oxygen production continued after the sample was sterilized via 160 degree C heat. Scientists now really agree that the oxygen production that Viking noticed was an abiotic process. But that also leads to a potential opportunity as some scientists think we can make oxygen farms out of a system similar to that used on Viking itself.
Continue reading “Future Mars Explorers Could be Farming Oxygen From Landscapes Like This”Mars Explorers are Going to Need air, and Lots of it. Here’s a Technology That Might Help Them Breath Easy
In situ resource utilization (ISRU) is still a very early science. Therefore, the technology utilized in it could be improved upon. One such technology that created one of the most useful materials for ISRU (oxygen) is MOXIE – the Mars OXygen In-situ Resource Utilization Experiment. A small-scale model of a MOXIE was recently tested on the Perseverance last year. Its primary goal is to create oxygen out of the Martian atmosphere.
Continue reading “Mars Explorers are Going to Need air, and Lots of it. Here’s a Technology That Might Help Them Breath Easy”Nearby Supernovae Were Essential to Life on Earth
It’s almost impossible to comprehend a supernova explosion’s violent, destructive power. An exploding supernova can outshine its host galaxy for a few weeks or even months. That seems almost impossible when considering that a galaxy can contain hundreds of billions of stars. Any planet too close to a supernova would be completely sterilized by all the energy released, its atmosphere would be stripped away, and it may even be shredded into pieces.
But like many things in nature, it all comes down to dose.
A certain amount of supernova activity might be necessary for life to exist.
Continue reading “Nearby Supernovae Were Essential to Life on Earth”The Early Earth was Really Horrible for Life
Earth has had a long and complex history since its formation roughly 4.5 billion years ago. Initially, it was a molten ball, but eventually, it cooled and became differentiated. The Moon formed from a collision between Earth and a protoplanet named Theia (probably), the oceans formed, and at some point in time, about 4 billion years ago, simple life appeared.
Those are the broad strokes, and scientists have worked hard to fill in a detailed timeline of Earth’s history. But there are a host of significant and poorly-understood periods in the timeline, lined up like targets for the scientific method. One of them concerns UV radiation and its effects on early life.
A new study probes the effects of UV radiation on Earth’s early life-forms and how it might have shaped our world.
Continue reading “The Early Earth was Really Horrible for Life”Chefs on the Moon Will be Cooking up Rocks to Make air and Water
NASA has delayed their Artemis mission to the Moon, but that doesn’t mean a return to the Moon isn’t imminent. Space agencies around the world have their sights set on our rocky satellite. No matter who gets there, if they’re planning for a sustained presence on the Moon, they’ll require in-situ resources.
Oxygen and water are at the top of a list of resources that astronauts will need on the Moon. A team of engineers and scientists are figuring out how to cook Moon rocks and get vital oxygen and water from them. They presented their results at the Europlanet Science Congress 2021.
Continue reading “Chefs on the Moon Will be Cooking up Rocks to Make air and Water”A Billion Years From now There won’t be Much Oxygen in the Earth’s Atmosphere
Breathe it while you still can. A new research study forecasts the future of oxygen in the Earth’s atmosphere and finds grim news. As the sun continues to warm, carbon dioxide will bind to rocks. This will starve plants, and in as little as a billion years they won’t be able to produce enough oxygen to keep our planet habitable (for us).
Continue reading “A Billion Years From now There won’t be Much Oxygen in the Earth’s Atmosphere”Earth’s Oxygen Could be Making the Moon Rust
It takes oxygen to make iron rust. So when scientists discovered hematite spread widely through lunar high latitudes, they were surprised. How did that happen?
A new study suggests that oxygen from Earth could be playing a role in rusting the Moon.
Continue reading “Earth’s Oxygen Could be Making the Moon Rust”New Data Show How Phytoplankton Pumps Carbon Out of the Atmosphere at an Enormous Scale
One of the most fascinating things about planet Earth is the way that life shapes the Earth and the Earth shapes life. We only have to look back to the Great Oxygenation Event (GOE) of 2.4 billion years ago to see how lifeforms have shaped the Earth. In that event, phytoplanktons called cyanobacteria pumped the atmosphere with oxygen, extinguishing most life on Earth, and paving the way for the development of multicellular life.
Early Earth satisfied the initial conditions for life to appear, and now, lifeforms shape the atmosphere, the landscape, and the oceans in many different ways.
At the base of many of these changes is phytoplankton.
Continue reading “New Data Show How Phytoplankton Pumps Carbon Out of the Atmosphere at an Enormous Scale”Molecular Oxygen on Mars is Behaving Unusually Through the Seasons. A Sign of Life?
An atmospheric drama has been playing out on Mars lately. Up until now, the main actor has been methane, and its unusual, spiking behaviour. But now Oxygen is taking the stage, and performing some theatrics of its own.
Continue reading “Molecular Oxygen on Mars is Behaving Unusually Through the Seasons. A Sign of Life?”Even if Exoplanets Have Atmospheres With Oxygen, it Doesn’t Mean There’s Life There

In their efforts to find evidence of life beyond our Solar System, scientists are forced to take what is known as the “low-hanging fruit” approach. Basically, this comes down to determining if planets could be “potentially habitable” based on whether or not they would be warm enough to have liquid water on their surfaces and dense atmospheres with enough oxygen.
This is a consequence of the fact that existing methods for examining distant planets are largely indirect and that Earth is only one planet we know of that is capable of supporting life. But what if planets that have plenty of oxygen are not guaranteed to produce life? According to a new study by a team from Johns Hopkins University, this may very well be the case.