One would think that crafting a shield out of water wouldn’t do much good (not in medieval combat re-enactments, anyways). But that’s precisely what the molecules in the early Solar System – some of the same ones that you are made out of today, perhaps – may have done. In their case, protection from broadswords wasn’t as much of a concern as the effects of ultraviolet radiation from the Sun.
UV light is pretty hard on molecules because it readily breaks them up into their constituent parts. Larger organic molecules that coalesced in the dusty disk out of which our planets formed billions of years ago would have been broken apart by the Sun’s rays, but calculations by two astronomers at the University of Michigan show that thousands of oceans worth of water present in a protoplanetary disk can shield other molecules from being broken up.
Edwin (Ted) Bergin and Thomas Bethell, both of the Department of Astronomy at the University of Michigan, calculated that in Sun-like systems the abundance of water early on can absorb much of the ultraviolet light from the central star. By shielding other molecules from being broken up, they continue to persist in the later stages of the disk’s development. In other words, these molecules hang around until the formation of planetesimals and planets, and this mechanism could have been guarded the constituents of life from the ravages of the Sun in our own Solar System.
Circumstellar disks modeled by Bergin and Bethell in their paper include DR Tau, AS 205A and AA Tau.
Bergin told Universe Today, “At present there have been upwards of 4 systems with water vapor observed. All are consistent with our model. I understand that there are numerous other detections of water vapor by Spitzer but these have yet to be published. The water vapor that we see is continually replenished by high temperature chemistry in these systems, so you would not see any degradation.”
In systems like the Solar System, planets form out of a disk of dust and gas that surrounds the young star. This large, flat disk later solidifies into planets, comets and asteroids. Near the center of the disk, between 1 and 5 astronomical units, warm water vapor in the disk could “protect” molecules inside this layer from being broken apart by UV light.
H2O breaks down when exposed to UV light into hydrogen and hydroxide. The hydroxide can be further broken down into oxygen and hydrogen atoms. But water, unlike other molecules, reforms at a quick pace, replenishing the shield of water vapor.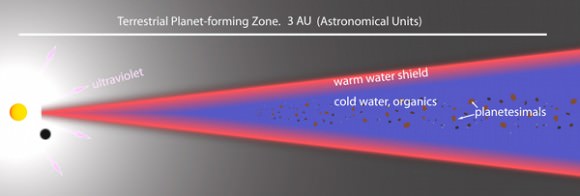
Smaller dust grains within the disk capture some of the UV radiation in the early formation periods of a protoplanetary disk. Once these dust grains start to snowball into bigger pieces, though, the UV light filters through and breaks apart molecules in the inner portions of the disk, where planets are in their early stages of formation.
The previous model for how organic molecules persisted past this point suggested that comets from the outer portion of the disk somehow fall into the center, releasing water to absorb the harmful radiation. But this model didn’t explain the hydroxide measurements for the disks so far observed.
If enough water is present, which seems to be the case in a handful of disks observed by the Spitzer Space Telescope, these other molecules remain intact, and as a bonus the water present in the interior portions of the disk also sticks around.
Bergin told Universe Today, “There are other molecules that can shield themselves – CO and H2 – but these cannot shield other molecules as well (because they capture only a fraction of the spectrum of light). Water is the only one with a strong formation that can compensate for destruction. It then provides the full shielding for other species. It is unlikely that another molecule will do this.”
This mechanism would only protect water vapor and other molecules in the inner part of the disk, closest to the star.
“This will likely be active in the inner few AU — at some point say between 5-10 AU it will become inactive and things will be inhospitable for various species [of molecule],” Bergin said.
So, where does all of the water go once the planets form? The vapor closest to the star – within about 1 AU – eventually gets broken down by the starlight into hydrogen and oxygen. At about 3 AU from the star, the water could constitute part of the planets and asteroids that form in that region. It may have been such asteroids that carried water to the surface of the Earth during its early formation, filling up our oceans. Outside of this region, H2O is broken down into hydrogen and oxygen and blown into space, said Bergin.
When asked whether this protective shield of water was present in our own Solar System, Bergin answered, “When we say that there were thousands of oceans of water vapor in the habitable zone, we mean around Sun-like stars. Presumably this was present around our Sun as well.”


That’s interesting. I have some scattered reactions.
First, I didn’t know that (as the PhysOrg article claims) our ocean’s water isotope ratios shows that it had to form further out than the Earth. But IIRC last year there was this paper on ratios from mantle processes showing that our volatiles must have come later than with the planetoid’s differentiation. So they are a Last Heavy Bombardment result, I take it?
Second, AFAIU photo-selection under radiation, foremost UV, seems to be an important process in building complex organics. I might be mistaken, but I believe that is why PAH forms on dust. But in any case both nucleotides and the biological assay of amino acids (AAs) shows signs of photo-selection. [“On the origin of life […]”, Mulkidjanian, and its ref:s.]
We seem to be able to assume that photo-selection is beneficial for life, not harmful. It drives pro-biological and proto-biological aggregation and complexity on surfaces everywhere (dust, planets). And it drives selection for some of the first proto-biological functionality. Here, dissipating energy quickly.
Third, this biologically beneficial photo-selection seems to have taken place late in life. Assuming a previous RNA world, AAs were chosen at the latest, but likeliest, when they were recruited for polymer formation to proteins.
Speaking of later times: “Ciesla is somewhat skeptical. He thinks the situation that the authors are considering occurred too late in the evolution of the solar system to explain Earth’s water. By the time the water-shielding mechanism becomes important, the solid material would have developed into clumps of rock, resulting in less overall surface area onto which water could condense.” [PhysOrg]
[And speaking of the RNA world, I was _blown away_ yesterday when I happened on a paper, from my alma mater no less, that indicates that we may have fossil traces reaching back _before_ it.
The ribosome is considered containing living fossils from the RNA world, as it builds proteins from RNA-RNA interaction. And the ribosome RNA (rRNA) was considered a ribozyme, a bona fide enzyme.
Turns out it isn’t really. By observation people have pared down the preserved reaction center as both containing essential water molecules and being a surface catalyst. A rather unusual but apparently not unknown trait. What is perhaps unusual is that the rRNA by simulations fitting observations is only a scaffold. If the model bears out to be a fact, the RNA holds and positions precisely 2 water molecules that are the catalyst and the reaction engine respectively.
The rRNA is trapping the water molecules simply by entropic forces. Usually RNA is, I believe, a lousy enzyme even when helped by metal ions at the right concentrations. Which is a problem for explaining RNA world metabolism, and how it bootstrapped itself to be king of the world. But water is a readily available proto-biological ion, and surface pockets are readily available proto-biological reaction sites.
Maybe these water-and-surface pocket “enzymes” are fossils from an important form of RNA world ribozyme activity. But also and more importantly – they could be fossils from a previous proto-biological surface metabolic world that build and incorporated RNA in the first place!
Interestingly too, the water reaction engine isn’t simply an acid-base electron donor AFAIU. It uses proton exchange instead, as water is want to do. That too may point the way to early biological contingency. As in the choice of, and now ubiquity of, proton exchange. It seems to have started way before membranes and cells. Maybe today’s protein enzymes just jumped on the band wagon when taking over and later elaborating previous function!
[“The transition state for peptide bond formation reveals the ribosome as a water trap”, Göran Wallin and Johan Åqvist]]
Oh, duh!
First, “showing that our volatiles must have come” – showing that the majority of our volatiles must have come.
Second, Ciesla isn’t really responding to Bergin here. My bad!