[/caption]
In late September, a team of scientists announced finding water molecule signatures across much of the Moon’s surface. Now, a second instrument on board India’s Chandrayaan-1’s lunar orbiter confirms how the water is being produced. The Sub keV Atom reflecting Analyzer (SARA) corroborates that electrically charged particles from the Sun interact with the oxygen present in some dust grains on the lunar surface to produce water. But the results bring out a new mystery of why some protons get reflected and not absorbed.
Scientists likened the Moon’s surface to a big sponge that absorbs the electrically charged particles. The lunar surface is a loose collection of irregular dust grains, or regolith, and the incoming charged particles should be trapped in the spaces between the grains and absorbed. When this happens to protons they are expected to interact with the oxygen in the lunar regolith to produce hydroxyl and water.
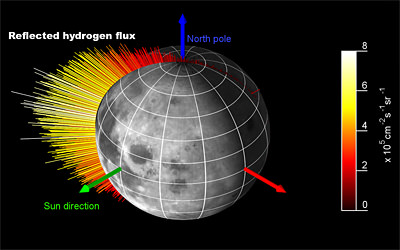
The SARA results confirm findings from Chandrayaan-1’s Moon Mineralogy Mapper (M3) that solar hydrogen nuclei are indeed being absorbed by the lunar regolith; however SARA data show that not every proton is absorbed. One out of every five rebounds into space. In the process, the proton joins with an electron to become an atom of hydrogen.
“We didn’t expect to see this at all,” says Stas Barabash, Swedish Institute of Space Physics, who is the European Principal Investigator for SARA.

Although Barabash and his colleagues do not know what is causing the reflections, the discovery paves the way for a new type of image to be made. Unfortunately, since the Chandrayaan-1 orbiter is no longer functioning, new data can’t be taken. However, the team can work with data already collected to further study the process.
The hydrogen shoots off with speeds of around 200 km/s and escapes without being deflected by the Moon’s weak gravity. Hydrogen is also electrically neutral, and is not diverted by the magnetic fields in space. So the atoms fly in straight lines, just like photons of light. In principle, each atom can be traced back to its origin and an image of the surface can be made. The areas that emit most hydrogen will show up the brightest.
While the Moon does not generate a global magnetic field, some lunar rocks are magnetized. Barabash and his team are currently creating images from collected data, to look for such ‘magnetic anomalies’ in lunar rocks. These generate magnetic bubbles that deflect incoming protons away into surrounding regions making magnetic rocks appear dark in a hydrogen image.
The incoming protons are part of the solar wind, a constant stream of particles given off by the Sun. They collide with every celestial object in the Solar System but are usually stopped by the body’s atmosphere. On bodies without such a natural shield, for example asteroids or the planet Mercury, the solar wind reaches the ground. The SARA team expects that these objects too will reflect many of the incoming protons back into space as hydrogen atoms.
Scientists with the ESA’s BepiColombo mission to Mercury are hoping to study the interaction between charged particles and the surface of Mercury. The spacecraft will be carrying two similar instruments to SARA and may find that the inner-most planet is reflecting more hydrogen than the Moon because the solar wind is more concentrated closer to the Sun.
Source: ESA

I don’t get this.
For example, in sputtering processes the knock-on atom (in the typical model being neutralized by electrons jumping or tunneling over the material potential barrier as the ion approach) would make a random walk process in the material while trading off energy to it and get target material moving. Some amounts of incoming material will end up surprisingly close to the surface or even leaving it.
The “sputtering yield” of the target material itself is typically 0.5 – 1 (50 – 100 %) with a max for steep incidence angles typically 2-5 times that. [Ar on Si, “Handbook of Plasma Processing Technology”, Rossnagel, Cuomo, Westwood.] The knock-on atom is of course but a small part of this flow. And hydrogen sucks as a sputterer due to the mass mismatch with, say, regolith atoms. Its sputter yield on Si is just a few percent.
Instead the reflection coefficient for lower energies is high at ~ 0.4 – 0.6.
Now I don’t know if the energy distributions of incoming ions, so I don’t know how many would be in the typical reflection region (up to say 100 eV for hydrogen) and in the typical sputtering region (up to a few keV). Unless I’m mistaken most of those hydrogen atoms have a kinetic energy of ~ 200 eV. Wouldn’t that be in the neighborhood of 0.2 reflection, perchance?
I’m not as knowledgable as Tobjorn but the LCROSS impact detected a Sodium signature so if we have Oxygen in the regolith and Hydrogen ions capable of combining we have NaOH, sodium hydroxide – drain cleaner. Handy for making soap and scrubbing CO2 from your atmosphere but if I remember my chemistry it doesn’t give up its OH lightly.