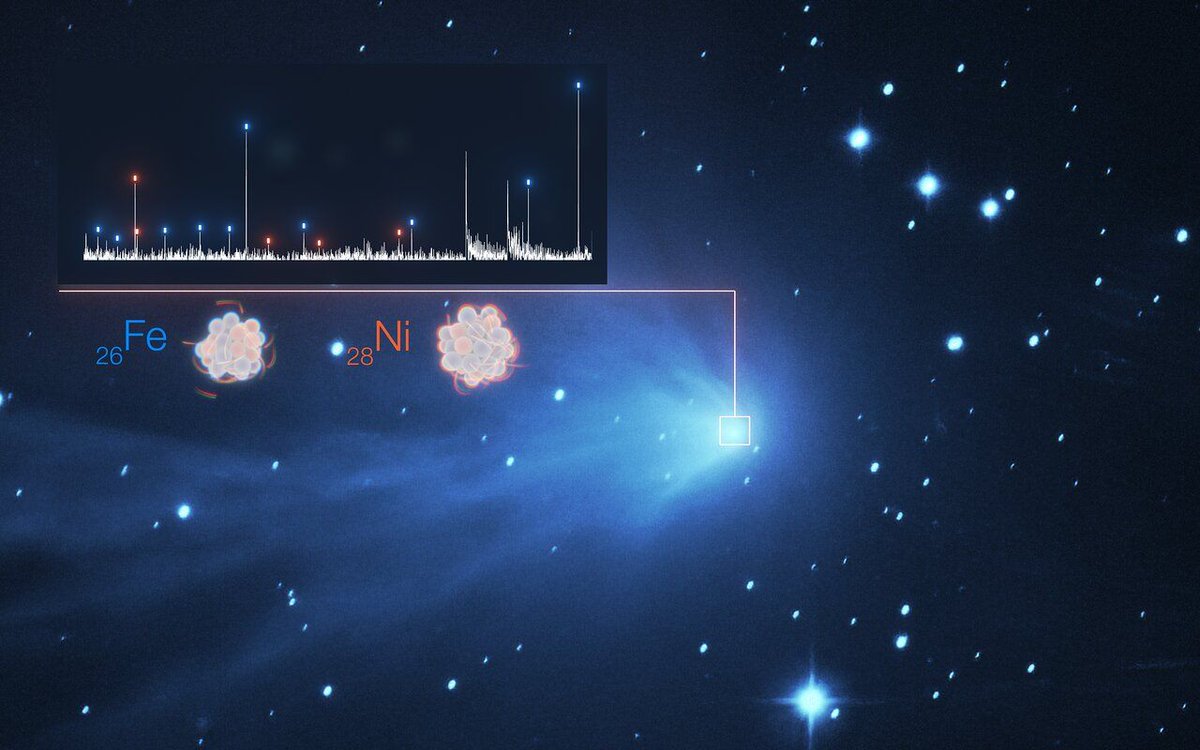
In outer space, an object’s location has a huge impact on its temperature. The closer the object is to its star, the hotter it most likely is. Heat then plays a major role in what materials are present in that object’s atmosphere, if it has one. Lighter elements such as hydrogen and helium and much easier to take a gaseous state and create an atmosphere. So it came as a surprise when two different teams found much heavier elements in the atmosphere of comets that were relatively far away from the Sun. And one of those comets happened to be from another solar system.
Continue reading “Comets Have Tails of gas, Dust… and Metal?”NASA Halts Work on its New Nuclear Generator for Deep Space Exploration
Another blow was dealt to deep space exploration this past weekend. The announcement comes from Jim Green, NASA’s Planetary Science Division Director. The statement outlines some key changes in NASA’s radioisotope program, and will have implications for the future exploration of the outer solar system.

We’ve written about the impending plutonium shortage and what it means for the future of spaceflight, as well as the recent restart of plutonium production. NASA is the only space agency that has conducted missions to the outer planets — even the European Space Agency’s Huygens lander had to hitch a ride with Cassini to get to Titan — and plutonium made this exploration possible. Continue reading “NASA Halts Work on its New Nuclear Generator for Deep Space Exploration”
U.S. To Restart Plutonium Production for Deep Space Exploration
The end of NASA’s plutonium shortage may be in sight. On Monday March 18th, NASA’s planetary science division head Jim Green announced that production of Plutonium-238 (Pu-238) by the United States Department of Energy (DOE) is currently in the test phases leading up to a restart of full scale production.
“By the end of the calendar year, we’ll have a complete plan from the Department of Energy on how they’ll be able to satisfy our requirement of 1.5 to 2 kilograms a year.” Green said at the 44th Lunar and Planetary Science Conference being held in Woodlands, Texas this past Monday.
This news comes none too soon. We’ve written previously on the impending Plutonium shortage and the consequences it has for future deep space exploration. Solar power is adequate in most cases when you explore the inner solar system, but when you venture out beyond the asteroid belt, you need nuclear power to do it.
Production of the isotope Pu-238 was a fortunate consequence of the Cold War. First produced by Glen Seaborg in 1940, the weapons grade isotope of plutonium (-239) is produced via bombarding neptunium (which itself is a decay product of uranium-238) with neutrons. Use the same target isotope of Neptunium-237 in a fast reactor, and Pu-238 is the result. Pu-238 produces 280x times the decay heat at 560 watts per kilogram versus weapons grade Pu-239 and is ideal as a compact source of energy for deep space exploration.
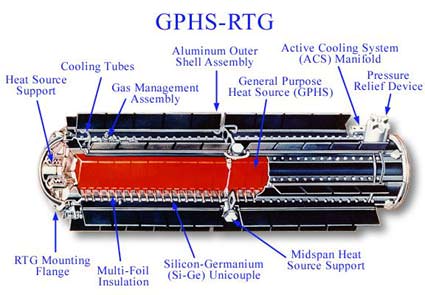
Since 1961, over 26 U.S. spacecraft have been launched carrying Multi-Mission Radioisotope Thermoelectric Generators (MMRTG, or formerly simply RTGs) as power sources and have explored every planet except Mercury. RTGs were used by the Apollo Lunar Surface Experiments Package (ALSEP) science payloads left on by the astronauts on the Moon, and Cassini, Mars Curiosity and New Horizons enroute to explore Pluto in July 2015 are all nuclear powered.
Plutonium powered RTGs are the only technology that we have currently in use that can carry out deep space exploration. NASA’s Juno spacecraft will be the first to reach Jupiter in 2016 without the use of a nuclear-powered RTG, but it will need to employ 3 enormous 2.7 x 8.9 metre solar panels to do it.
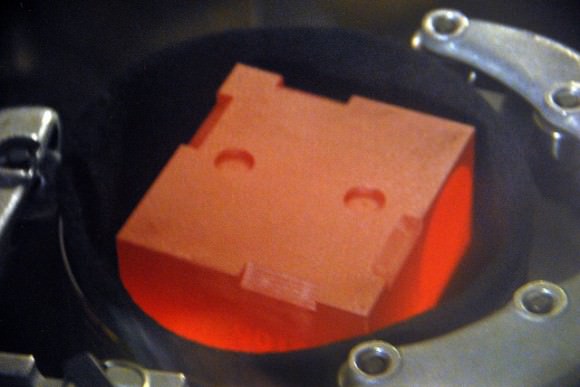
The problem is, plutonium production in the U.S. ceased in 1988 with the end of the Cold War. How much Plutonium-238 NASA and the DOE has stockpiled is classified, but it has been speculated that it has at most enough for one more large Flag Ship class mission and perhaps a small Scout class mission. Plus, once weapons grade plutonium-239 is manufactured, there’s no re-processing it the desired Pu-238 isotope. The plutonium that currently powers Curiosity across the surface of Mars was bought from the Russians, and that source ended in 2010. New Horizons is equipped with a spare MMRTG that was built for Cassini, which was launched in 1999.

As an added bonus, plutonium powered missions often exceed expectations as well. For example, the Voyager 1 & 2 spacecraft had an original mission duration of five years and are now expected to continue well into their fifth decade of operation. Mars Curiosity doesn’t suffer from the issues of “dusty solar panels” that plagued Spirit and Opportunity and can operate through the long Martian winter. Incidentally, while the Spirit and Opportunity rovers were not nuclear powered, they did employ tiny pellets of plutonium oxide in their joints to stay warm, as well as radioactive curium to provide neutron sources in their spectrometers. It’s even quite possible that any alien intelligence stumbles upon the five spacecraft escaping our solar system (Pioneer 10 & 11, Voyagers 1 & 2, and New Horizons) could conceivably date their departure from Earth by measuring the decay of their plutonium power source. (Pu-238 has a half life of 87.7 years and eventually decays after transitioning through a long series of daughter isotopes into lead-206).

The current production run of Pu-238 will be carried out at the Oak Ridge National Laboratory (ORNL) using its High Flux Isotope Reactor (HFIR). “Old” Pu-238 can also be revived by adding newly manufactured Pu-238 to it.
“For every 1 kilogram, we really revive two kilograms of the older plutonium by mixing it… it’s a critical part of our process to be able to utilize our existing supply at the energy density we want it,” Green told a recent Mars exploration planning committee.
Still, full target production of 1.5 kilograms per year may be some time off. For context, the Mars rover Curiosity utilizes 4.8 kilograms of Pu-238, and New Horizons contains 11 kilograms. No missions to the outer planets have left Earth since the launch of Curiosity in November 2011, and the next mission likely to sport an RTG is the proposed Mars 2020 rover. Ideas on the drawing board such as a Titan lake lander and a Jupiter Icy Moons mission would all be nuclear powered.

Along with new plutonium production, NASA plans to have two new RTGs dubbed Advanced Stirling Radioisotope Generators (ASRGs) available by 2016. While more efficient, the ASRG may not always be the device of choice. For example, Curiosity uses its MMRTG waste heat to keep instruments warm via Freon circulation. Curiosity also had to vent waste heat produced by the 110-watt generator while cooped up in its aero shell enroute to Mars.
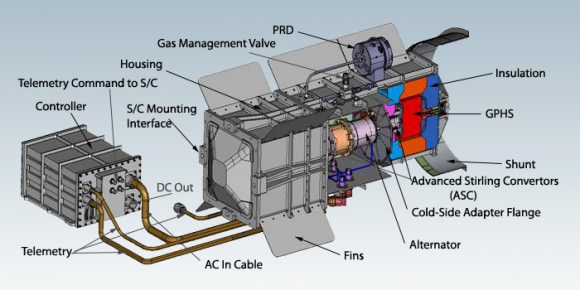
And of course, there are the added precautions that come with launching a nuclear payload. The President of the United States had to sign off on the launch of Curiosity from the Florida Space Coast. The launch of Cassini, New Horizons, and Curiosity all drew a scattering of protesters, as does anything nuclear related. Never mind that coal fired power plants produce radioactive polonium, radon and thorium as an undesired by-product daily.

Said launches aren’t without hazards, albeit with risks that can be mitigated and managed. One of the most notorious space-related nuclear accidents occurred early in the U.S. space program with the loss of an RTG-equipped Transit-5BN-3 satellite off of the coast of Madagascar shortly after launch in 1964. And when Apollo 13 had to abort and return to Earth, the astronauts were directed to ditch the Aquarius Landing Module along with its nuclear-powered science experiments meant for the surface of the Moon in the Pacific Ocean near the island of Fiji. (They don’t tell you that in the movie) One wonders if it would be cost effective to “resurrect” this RTG from the ocean floor for a future space mission. On previous nuclear-equipped launches such as New Horizons, NASA placed the chance of a “launch accident that could release plutonium” at 350-to-1 against Even then, the shielded RTG is “over-engineered” to survive an explosion and impact with the water.
But the risks are worth the gain in terms of new solar system discoveries. In a brave new future of space exploration, the restart of plutonium production for peaceful purposes gives us hope. To paraphrase Carl Sagan, space travel is one of the best uses of nuclear fission that we can think of!
No Nukes? NASA’s Plutonium Production Predicament
[/caption]
Mars Science Laboratory, launched three days ago on the morning of Saturday, November 26, is currently on its way to the Red Planet – a journey that will take nearly nine months. When it arrives the first week of August 2012, MSL will begin investigating the soil and atmosphere within Gale Crater, searching for the faintest hints of past life. And unlike the previous rovers which ran on solar energy, MSL will be nuclear-powered, generating its energy through the decay of nearly 8 pounds of plutonium-238. This will potentially keep the next-generation rover running for years… but what will fuel future exploration missions now that NASA may no longer be able to fund the production of plutonium?
Pu-238 is a non-weapons-grade isotope of the radioactive element, used by NASA for over 50 years to fuel exploration spacecraft. Voyagers, Galileo, Cassini… all had radioisotope thermoelectric generators (RTGs) that generated power via Pu-238. But the substance has not been in production in the US since the late 1980s; all Pu-238 has since been produced in Russia. But now there’s only enough left for one or two more missions and the 2012 budget plan does not yet allot funding for the Department of Energy to continue production.
Where will future fuel come from? How will NASA power its next lineup of robotic explorers? (And why aren’t more people concerned about this?)
Amateur astronomer, teacher and blogger David Dickinson went into detail about this conundrum in an informative article written earlier this year. Here are some excerpts from his post:
________________
When leaving our fair planet, mass is everything. Space being a harsh place, you must bring nearly everything you need, including fuel, with you. And yes, more fuel means more mass, means more fuel, means… well, you get the idea. One way around this is to use available solar energy for power generation, but this only works well in the inner solar system. Take a look at the solar panels on the Juno spacecraft bound for Jupiter next month… those things have to be huge in order to take advantage of the relatively feeble solar wattage available to it… this is all because of our friend the inverse square law which governs all things electromagnetic, light included.

To operate in the environs of deep space, you need a dependable power source. To compound problems, any prospective surface operations on the Moon or Mars must be able to utilize energy for long periods of sun-less operation; a lunar outpost would face nights that are about two Earth weeks long, for example. To this end, NASA has historically used Radioisotope Thermal Generators (RTGs) as an electric “power plant” for long term space missions. These provide a lightweight, long-term source of fuel, generating from 20-300 watts of electricity. Most are about the size of a small person, and the first prototypes flew on the Transit-4A & 5BN1/2 spacecraft in the early 60’s. The Pioneer, Voyager, New Horizons, Galileo and Cassini spacecraft all sport Pu238 powered RTGs. The Viking 1 and 2 spacecraft also had RTGs, as did the long term Apollo Lunar Surface Experiments Package (ALSEP) experiments that Apollo astronauts placed on the Moon. An ambitious sample return mission to the planet Pluto was even proposed in 2003 that would have utilized a small nuclear engine.
Video: what is plutonium really like?

David goes on to mention the undeniable dangers of plutonium…
Plutonium is nasty stuff. It is a strong alpha-emitter and a highly toxic metal. If inhaled, it exposes lung tissue to a very high local radiation dose with the attending risk of cancer. If ingested, some forms of plutonium accumulate in our bones where it can damage the body’s blood-forming mechanism and wreck havoc with DNA. NASA had historically pegged a chance of a launch failure of the New Horizons spacecraft at 350-to-1 against, which even then wouldn’t necessarily rupture the RTG and release the contained 11 kilograms of plutonium dioxide into the environment. Sampling conducted around the South Pacific resting place of the aforementioned Apollo 13 LM re-entry of the ascent stage of the Lunar Module, for example, suggests that the reentry of the RTG did NOT rupture the container, as no plutonium contamination has ever been found.
Yet the dangers of nuclear power often overshadow its relative safety and unmistakable benefit:
The black swan events such as Three Mile Island, Chernobyl and Fukushima have served to demonize all things nuclear, much like the view that 19thcentury citizens had of electricity. Never mind that coal-fired plants put many times the equivalent of radioactive contamination into the atmosphere in the form of lead210, polonium214, thorium and radon gases, every day. Safety detectors at nuclear plants are often triggered during temperature inversions due to nearby coal plant emissions… radiation was part of our environment even before the Cold War and is here to stay. To quote Carl Sagan, “Space travel is one of the best uses of nuclear weapons that I can think of…”
Yet here we are, with a definite end in sight to the supply of nuclear “weapons” needed to power space travel…
Currently, NASA faces a dilemma that will put a severe damper on outer solar system exploration in the coming decade. As mentioned, current plutonium reserves stand at about enough for the Mars Science Laboratory Curiosity, which will contain 4.8kilograms of plutonium dioxide, and one last large & and perhaps one small outer solar system mission. MSL utilizes a new generation MMRTG (the “MM” stands for Multi-Mission) designed by Boeing that will produce 125 watts for up to 14 years. But the production of new plutonium would be difficult. Restart of the plutonium supply-line would be a lengthy process, and take perhaps a decade. Other nuclear based alternatives do indeed exist, but not without a penalty either in low thermal activity, volatility, expense in production, or short half life.
The implications of this factor may be grim for both manned and unmanned space travel to the outer solar system. Juxtaposed against at what the recent 2011 Decadal Survey for Planetary Exploration proposes, we’ll be lucky to see many of those ambitious “Battlestar Galactica” –style outer solar system missions come to pass.
Landers, blimps and submersibles on Europa, Titan, and Enceladus will all operate well out of the Sun’s domain and will need said nuclear power plants to get the job done… contrast this with the European Space Agency’s Huygens probe, which landed on Titan after being released from NASA’s Cassini spacecraft in 2004, which operated for scant hours on battery power before succumbing to the -179.5 C° temps that represent a nice balmy day on the Saturnian moon.
So, what’s a space-faring civilization to do? Certainly, the “not going into space” option is not one we want on the table, and warp or Faster-Than-Light drives a la every bad science fiction flick are nowhere in the immediate future. In [my] highly opinionated view, NASA has the following options:
Exploit other RTG sources at penalty. As mentioned previously, other nuclear sources in the form of Plutonium, Thorium, and Curium isotopes do exist and could be conceivably incorporated into RTGs; all, however, have problems. Some have unfavorable half-lives; others release too little energy or hazardous penetrating gamma-rays. Plutonium238 has high energy output throughout an appreciable life span, and its alpha particle emissions can be easily contained.
Design innovative new technologies. Solar cell technology has come a long way in recent years, making perhaps exploration out to the orbit of Jupiter is do-able with enough collection area. The plucky Spirit and Opportunity Mars rovers(which did contain Curium isotopes in their spectrometers!) made do well past their respective warranty dates using solar cells, and NASA’s Dawn spacecraft currently orbiting the asteroid Vesta sports an innovative ion-drive technology.
Push to restart plutonium production. Again, it is not that likely or even feasible that this will come to pass in today’s financially strapped post-Cold War environment. Other countries, such as India and China are looking to “go nuclear” to break their dependence on oil, but it would take some time for any trickle-down plutonium to reach the launch pad. Also, power reactors are not good producers of Pu238. The dedicated production of Pu238 requires either high neutron flux reactors or specialized “fast” reactors specifically designed for the production of trans-uranium isotopes…
Based on the realities of nuclear materials production the levels of funding for Pu238 production restart are frighteningly small. NASA must rely on the DOE for the infrastructure and knowledge necessary and solutions to the problem must fit the realities within both agencies.
And that’s the grim reality of a brave new plutonium-free world that faces NASA; perhaps the solution will come as a combination of some or all of the above. The next decade will be fraught with crisis and opportunity… plutonium gives us a kind of Promethean bargain with its use; we can either build weapons and kill ourselves with it, or we can inherit the stars.
Thanks to David Dickinson for the use of his excellent article; be sure to read the full version on his Astro Guyz site here (and follow David on Twitter @astroguyz.) Also check out this article by Emily Lakdawalla of The Planetary Society on how the RTG unit for Curiosity was made.
“There are some people who legitimately feel like this is simply not a priority, that there’s not enough money and it’s not their problem. But I think if you try to step back and look at the forest and not just the individual trees, this is one of the things that has helped drive us to become a technological powerhouse. What we’ve done with robotic space exploration is something that people not just in the U.S., but around the world, can look up to.”
– Ralph McNutt, planetary scientist at Johns Hopkins University’s Applied Physics Laboratory (APL)
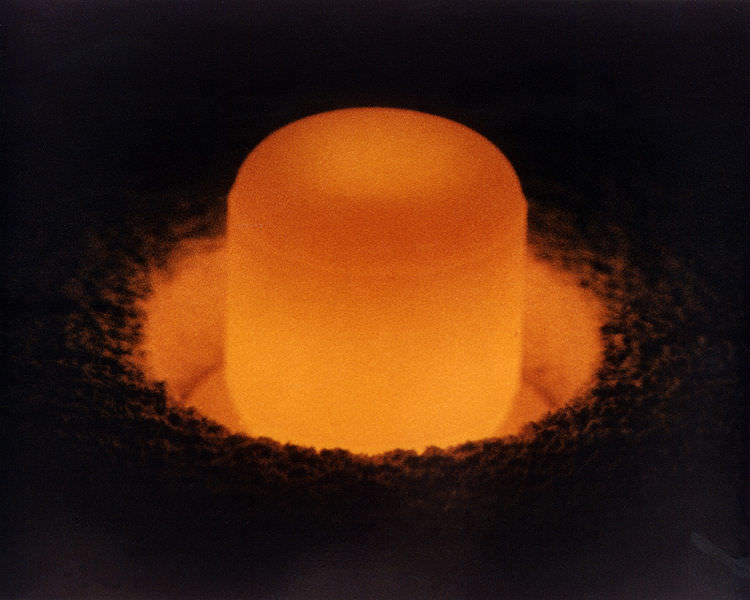
( Top image credit © 2011 Theodore Gray periodictable.com; used with permission.)
What is Plutonium?
[/caption]
The name itself conjures up imagines of mini nukes and sophisticated space-age gadgets doesn’t it? Well for some people it does. For others, Plutonium (Pu, atomic number of 94 on the periodic table of elements) spawns images of nuclear reactors, atomic energy and nuclear waste. All of these are true to an extent, but the reality behind this radioactive element is understandably more complex. For starters, plutonium is a silvery white actinide metal that is radioactive, and hence quite dangerous when exposed to living tissue. It is one of the key ingredients in the making of atomic weapons, but is also produced in nuclear reactors as a result of slow fission. There are also several isotopes of the element, but for our purposes, the most important is Plutonium-239, a fissile isotope that is used for both nuclear power and weapons and has a half-life of 24,100 years.
Plutonium-238 was first discovered as an element on Dec.14th1940, and then chemically identified on February 23rd 1941through the deuteron bombardment of Uranium in a cyclotron by Glenn T. Seaborg and his team of scientists, working out of the University of California in Berkley. The team submitted a paper publishing their findings; however, this paper was retracted when it became clear that Plutonium-239 was a fissile material that could be useful in the construction of an atomic weapon. At this time, the US was deep into the development of an atomic bomb (aka. the Manhattan Project) because it was believed that Germany was doing the same. For this reason, publication of Seaborg’s work was delayed until 1946, a year after the Second World War ended and security surrounding atomic research was no longer a concern. Seaborg decided to name the element after Pluto because of the recent discovery of element 93, Neptunium, and felt that element 94 should accordingly be named after the next planet in the Solar System.
Towards the end of WWII, two nuclear reactors were created which would produce the plutonium used in the construction of “Trinity”, “Fat Man” and other atomic weapons. These were the X-10 Graphite Reactor facility in Oak Ridge (which later became the Oak Ridge National Laboratory) and the Hanford B reactor (built in 1943 and 45 respectively). Large stockpiles were subsequently built up by the US and USSR during the Cold War, and have since become the focus of nuclear proliferation treaty concerns. Today, it is estimated that several tonnes of plutonium isotopes exist in our biosphere, the result of atomic testing during the 1950’s and 60’s.
We have written many articles about Plutonium for Universe Today. Here’s an article about Plutonium shortage in NASA, and here’s an article about Plutonium – 238.
If you’d like more info on Plutonium, check out Wikipedia – Plutonium, and here’s a link to World Nuclear page about Plutonium.
We’ve also recorded an entire episode of Astronomy Cast all about Nuclear Forces. Listen here, Episode 105: The Strong and Weak Nuclear Forces.
Sources:
http://en.wikipedia.org/wiki/Plutonium
http://www.world-nuclear.org/info/inf15.html
http://periodic.lanl.gov/elements/94.html
http://en.wikipedia.org/wiki/Nuclear_proliferation
http://en.wikipedia.org/wiki/Actinide
http://en.wikipedia.org/wiki/Cyclotron