In the beginning, the universe created three elements: hydrogen, helium, and lithium. There isn’t much you can do with these simple elements, other than to let gravity collapse them into stars, galaxies, and black holes. But stars have the power of alchemy. Within their hearts, they can fuse these elements into new ones. Carbon, nitrogen, oxygen, and others, all up to the heavy element of iron. When these first stars exploded, they scattered the new elements across the cosmos, creating planets, new stars, and even us.
Continue reading “Colliding Neutron Stars Don’t Make Enough Gold to Explain What We See in the Universe”Weekly Space Hangout – Jan. 8, 2016: Elizabeth S. Sexton-Kennedy from FermiLab
Host: Fraser Cain (@fcain)
Special Guest:Elizabeth S. Sexton-Kennedy, who works at FermiLab as Compact Muon Solenoid (CMS) Offline Coordinator. CMS (at CERN/LHC) is a particle detector that is designed to see a wide range of particles and phenomena produced in high-energy proton collisions in the LHC.
Guests:
Morgan Rehnberg (cosmicchatter.org / @MorganRehnberg )
Alessondra Springmann (@sondy)
Paul Sutter (pmsutter.com / @PaulMattSutter)
Dave Dickinson (@astroguyz / www.astroguyz.com)
Pamela Gay (cosmoquest.org / @cosmoquestx / @starstryder)
Continue reading “Weekly Space Hangout – Jan. 8, 2016: Elizabeth S. Sexton-Kennedy from FermiLab”
Can You Kill a Star With Iron?
Since the energy required to fuse iron is more than the energy that you get from doing it, could you use iron to kill a star like our sun?
A fan favorite was How Much Water Would it Take to Extinguish the Sun? Go ahead and watch it now if you like. Or… if you don’t have time to watch me set up the science, deliver a bunch of hilarious zingers and obscure sci-fi references, here’s the short version:
The Sun is not on fire, it’s a fusion reaction. Hydrogen mashes up to produce helium and energy. Lots and lots of energy. Water is mostly hydrogen, adding water would give more fuel and make it burn hotter. But some of you clever viewers proposed another way to kill the Sun. Kill it with iron!
Iron? That seems pretty specific. Why iron and not something else, like butter, donuts, or sitting on the couch playing video games – all the things working to kill me? Is iron poison to stars? An iron bar? Possibly iron bullets? Iron punches? Possibly from fashioning a suit and attacking it as some kind of Iron Man?
Time for some stellar physics. Stars are massive balls of plasma. Mostly hydrogen and helium, and leftover salad from the Big Bang. Mass holds them together in a sphere, creating temperatures and pressures at their cores, where atoms of hydrogen are crushed together into helium, releasing energy. This energy, in the form of photons pushes outward. As they escape the star, this counteracts the force of gravity trying to pull it inward.
Over the course of billions of years, the star uses up the reserves of hydrogen, building up helium. If it’s massive enough, it will switch to helium when the hydrogen is gone. Then it can switch to oxygen, and then silicon, and all the way up the periodic table of elements.
The most massive stars in the Universe, the ones with at least 8 times the mass of the Sun, have enough temperature and pressure that they can fuse elements all the way up to iron, the 26th element on the Periodic Table. At that point, the energy required to fuse iron is more than the energy that you get from fusing iron, no matter how massive a star you are.

In a fraction of a second, the core of the Sun shuts off. It’s no longer pushing outward with its light pressure, and so the outer layers collapse inward, creating a black hole and a supernova. It sure looks like the build up of iron in the core killed it.
Is it true then? Is iron the Achilles heel of stars? Not really. Iron is the byproduct of fusion within the most massive stars. Just like ash is the byproduct of combustion, or poop is the byproduct of human digestion.
It’s not poison, which stops or destroys processes within the human body. A better analogy might be fiber. Your body can’t get any nutritional value out of fiber, like grass. If all you had to eat was grass, you’d starve, but it’s not like the grass is poisoning you. As long as you got adequate nutrition, you could eat an immense amount of grass and not die. It’s about the food, not the grass.
The Sun already has plenty of iron; it’s 0.1% iron. That little nugget would work out to be 330 times the mass of the Earth. If you gave it much more iron, it would just give the Sun more mass, which would give it more gravity to raise the temperature and pressure at the core, which would help it do even more fusion.
If you just poured iron into a star, it wouldn’t kill it. It would just make it more massive and then hotter and capable of supporting the fusion of heavier elements. As long as there’s still viable fuel at the core of the star, and adequate temperatures and pressures, it’ll continue fusing and releasing energy.
If you could swap out the hydrogen in the Sun with a core of iron, you would indeed kill it dead, or any star for that matter. It wouldn’t explode, though. Only if it was at least 8 times the mass of the Sun to begin with. Then would you have enough mass bearing down on the inert core to create a core collapse supernova.
In fact, since you’ve got the power to magically replace stellar cores, you would only need to replace the Sun’s core with carbon or oxygen to kill it. It actually doesn’t have enough mass to fuse even carbon. As soon as you replaced the Sun’s core, it would shut off fusion. It would immediately become a white dwarf, and begin slowly cooling down to the background temperature of the Universe.
Iron in bullet, bar, man or any other form isn’t poison to a star. It just happens to be an element that no star can use to generate energy from fusion. As long as there’s still viable fuel at the core of a star, and the pressure and temperature to bring them together, the star will continue to pump out energy.
What other exotic ways would you use to try and kill the Sun? Give us your suggestions in the comments below.
Earth is the Most Exotic Place In The Universe
I’m often asked by students in my community education astronomy classes whether any new elements have been found in outer space unknown on Earth. The answer to the question is no – nature uses the same 98 natural elements to fashion everything from the familiar stars and planets to those in the farthest galaxies we can see. Outside of an occasional compound or mineral, Earth is the place where you’ll find more exotic elements than anywhere else in the universe.
An element is a pure substance made of just one type of atom. What sets one element apart from another is the number of protons in the nuclei of its atoms. Any atom with six protons will always be carbon, 79 protons gold, 94 protons plutonium and 1 proton hydrogen. The proton number is also the element’s atomic number on the periodic table of elements. Elements in the table are arranged according to their atomic number.
The two most common elements are hydrogen and helium, numbers 1 and 2 in the periodic table; together they make up 98% of all the visible matter in the universe. The remaining 2% includes everything else from lightweight lithium (number 3) all the way up to californium (98), the heaviest natural element found on Earth and in the stars. Californium is unstable and “decays” into simpler elements. Although scientists make it in the lab by bombarding berkelium (97) with neutrons, trace amounts of this very rare element are found naturally in rich uranium deposits.
When I was in high school studying chemistry, the periodic table of elements ended at Lawrencium (103). At present there are 118 elements, the most recent one created in the lab being ununoctium (you-nah-NOC-tee-um). Matter of fact, all the elements beyond 98 are artificial, brought to life in nuclear reactors or in particle accelerator experiments. They live very short lives. With so many positively-charged protons pushing against one another in their nuclei, these elements quickly break apart into simpler ones in a process called radioactive decay.

Back at the time of the Big Bang, when the universe sprang into existence, only the simplest elements – hydrogen, helium and trace amounts of lithium – were cooked up. You can’t build a planet from such fluffy stuff. It took the first generation of stars, which formed from these basic building blocks, to synthesize more complicated elements like carbon, oxygen, sulfur and the like via nuclear fusion in their cores.
When the stars exploded as supernovae, not only were these brand new elements blasted into space, but the enormous heat and pressure during the blast built even heavier elements like gold, copper, mercury and lead. All became incorporated in a second generation of stars. And a third.
The 2% of star-made elements, which include carbon, oxygen, nitrogen and silicon among others, went to build the planets and later became essential for life. We’re made of highly processed material you and I. The atoms of our beings have been in and out of the cores of several generations of stars. Think about this good and hard and you might just get in touch with your own “inner star”.
Let’s reframe the question about exotic materials in space not present on Earth. Instead of elements, if we look at compounds, we hit paydirt. A compound is also a pure substance but consists of two or more chemical elements joined together. Familiar compounds include water (two hydrogens joined to one oxygen) and salt (one sodium and one chlorine).
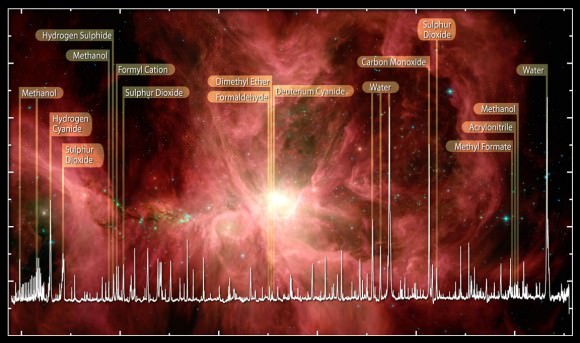
Astronomers have found about 220 compounds or molecules in outer space many of them with siblings on Earth but some alien. We don’t have to look far to find them since a few have been delivered right to our doorstep as rocky packages called meteorites. Here’s a short list of new minerals that formed within asteroids (where meteorites originate) under conditions very different from those found on Earth:
* Barringerite – a metallic compound made of iron, nickel and phosphorus
* Oldhamite – brown mineral made of calcium, magnesium and sulfur
* Kosmochlor – green mineral containing calcium, chromium, silicon and oxygen
How about new stuff on planets and comets? Astronomers have discovered compounds in the atmospheres of the giant planets Jupiter, Saturn, Uranus and Neptune like silane (silicon-hydrogen), arsine (arsenic-hydrogen) and phosphine (phosphorus-hydrogen) that don’t exist naturally on Earth. Humans have created all three in the lab and put them to good use in various industries including the manufacture of semi-conductors.

And then there’s Brownleeite, a manganese silicide found in 2003 in a dust particle shed by comet 26P/Grigg-Skjellerup. Moving beyond the solar system, astronomers see unusual long-chained carbon molecules in space that couldn’t form on Earth because oxygen would tear them apart. Space is their safe haven.
So, Earth is the location in the Universe where you’ll find more exotic elements than anywhere else. Thanks to human activity and the complicated molecules that wound together to form life, Earth’s the most exotic place in the universe.
Where is Uranium Located
[/caption]
Uranium is a silvery white metal and is number 92 on the table of periodic elements. It is a well-known element because of its radioactive properties which are used in nuclear reactor powered by nuclear fission. We know that this element is very sought after as source of power by many countries wanting to shift from oil and fossil fuel based economies. So where is Uranium located and how do miners harvest it?
To understand how it is found we need to learn about how it was discovered. Uranium was first discovered by German chemist martin Heinrich Klaproth in 1749 when he was heat treating Minerals. He named the new mineral produced Uranium. The first pure sample of Uranium metal was produced in 1841 by Eugène-Melchior Péligot an analytical chemist who was heat treating Uranium tetrachloride. Demand for Uranium outside its more mundane uses as a window dye was initiated by the discovery of its fissile nuclear properties by Enrico Fermi. Mr. Fermi would go on to lead the Manhattan Project in 1942 that lead to the creation of nuclear weapons and reactors. When the energy it produced was realized the demand for Uranium immediately increased.
So where is Uranium located? In space Uranium is formed naturally occurring in supernovas. However since we can’t even travel to the nearest star it is just a minor fact. On Earth Uranium is surprisingly plentiful for a heavy metal. In fact estimate place the Earth’s supply of Uranium at 30 times that of Silver. This is because Uranium can be found in topsoil anywhere on the planet as well as in the mantle. Scientist even theorize that the natural decay of Uranium and other radioactive elements is what heats the Earth’s core and mantle causing convection currents in the magma and creating plate tectonics.
Uranium can be found as part of a lot of different minerals such as uranite. The element rarely occurs in its pure form. Even then the more fissile kinds of isotopes aren’t plentiful in nature. Uranium ore is the main source of uranium even though with the discovery of how wide spread it is in the Earth’s crust and scientist are looking for inexpensive ways to process it from the soil. In the meanwhile Uranium ore can be found in mines in Canada, Russia, and in Sub-Saharan Africa.
We have written many articles about Uranium for Universe Today. Here’s an article about the lunar Uranium, and here’s an article about nuclear fission.
If you’d like more info on Uranium, check out Wikipedia, and here’s a link to the Encyclopedia of Earth.
We’ve also recorded an entire episode of Astronomy Cast all about the Atom. Listen here, Episode 164: Inside the Atom.
Sources:
Wikipedia
Encyclopedia of Earth
World Nuclear Association