Since the energy required to fuse iron is more than the energy that you get from doing it, could you use iron to kill a star like our sun?
A fan favorite was How Much Water Would it Take to Extinguish the Sun? Go ahead and watch it now if you like. Or… if you don’t have time to watch me set up the science, deliver a bunch of hilarious zingers and obscure sci-fi references, here’s the short version:
The Sun is not on fire, it’s a fusion reaction. Hydrogen mashes up to produce helium and energy. Lots and lots of energy. Water is mostly hydrogen, adding water would give more fuel and make it burn hotter. But some of you clever viewers proposed another way to kill the Sun. Kill it with iron!
Iron? That seems pretty specific. Why iron and not something else, like butter, donuts, or sitting on the couch playing video games – all the things working to kill me? Is iron poison to stars? An iron bar? Possibly iron bullets? Iron punches? Possibly from fashioning a suit and attacking it as some kind of Iron Man?
Time for some stellar physics. Stars are massive balls of plasma. Mostly hydrogen and helium, and leftover salad from the Big Bang. Mass holds them together in a sphere, creating temperatures and pressures at their cores, where atoms of hydrogen are crushed together into helium, releasing energy. This energy, in the form of photons pushes outward. As they escape the star, this counteracts the force of gravity trying to pull it inward.
Over the course of billions of years, the star uses up the reserves of hydrogen, building up helium. If it’s massive enough, it will switch to helium when the hydrogen is gone. Then it can switch to oxygen, and then silicon, and all the way up the periodic table of elements.
The most massive stars in the Universe, the ones with at least 8 times the mass of the Sun, have enough temperature and pressure that they can fuse elements all the way up to iron, the 26th element on the Periodic Table. At that point, the energy required to fuse iron is more than the energy that you get from fusing iron, no matter how massive a star you are.

In a fraction of a second, the core of the Sun shuts off. It’s no longer pushing outward with its light pressure, and so the outer layers collapse inward, creating a black hole and a supernova. It sure looks like the build up of iron in the core killed it.
Is it true then? Is iron the Achilles heel of stars? Not really. Iron is the byproduct of fusion within the most massive stars. Just like ash is the byproduct of combustion, or poop is the byproduct of human digestion.
It’s not poison, which stops or destroys processes within the human body. A better analogy might be fiber. Your body can’t get any nutritional value out of fiber, like grass. If all you had to eat was grass, you’d starve, but it’s not like the grass is poisoning you. As long as you got adequate nutrition, you could eat an immense amount of grass and not die. It’s about the food, not the grass.
The Sun already has plenty of iron; it’s 0.1% iron. That little nugget would work out to be 330 times the mass of the Earth. If you gave it much more iron, it would just give the Sun more mass, which would give it more gravity to raise the temperature and pressure at the core, which would help it do even more fusion.
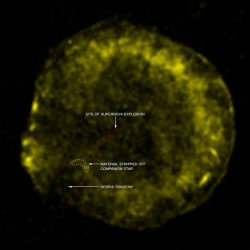
If you just poured iron into a star, it wouldn’t kill it. It would just make it more massive and then hotter and capable of supporting the fusion of heavier elements. As long as there’s still viable fuel at the core of the star, and adequate temperatures and pressures, it’ll continue fusing and releasing energy.
If you could swap out the hydrogen in the Sun with a core of iron, you would indeed kill it dead, or any star for that matter. It wouldn’t explode, though. Only if it was at least 8 times the mass of the Sun to begin with. Then would you have enough mass bearing down on the inert core to create a core collapse supernova.
In fact, since you’ve got the power to magically replace stellar cores, you would only need to replace the Sun’s core with carbon or oxygen to kill it. It actually doesn’t have enough mass to fuse even carbon. As soon as you replaced the Sun’s core, it would shut off fusion. It would immediately become a white dwarf, and begin slowly cooling down to the background temperature of the Universe.
Iron in bullet, bar, man or any other form isn’t poison to a star. It just happens to be an element that no star can use to generate energy from fusion. As long as there’s still viable fuel at the core of a star, and the pressure and temperature to bring them together, the star will continue to pump out energy.
What other exotic ways would you use to try and kill the Sun? Give us your suggestions in the comments below.


Fusion is kinda what big globs of hydrogen do, so to stop the sun, you’d have to make it stop being a big glob of hydrogen. You could always strip mass off the surface until it shrunk into a (really really hot) brown dwarf. I suppose you could spin up the sun until its core pressure dropped to brown dwarf levels, but that would mean some layer would be in orbit and everything outside of that would be ejected, so this is really just a fancy version of the mass removal idea.
You could (…somehow…) collapse some portion of the core into a black hole of sufficient size to not starve and evaporate at the ambient density of the core, but I think you went over that already. And if we’re getting really crazy, you could twiddle the various universal constants until conditions inside the sun were no longer sufficient to fuse hydrogen.
Say, suppose that you could take a cold, dark nebula and collapse it into a star without compressional heating. If you held it at 3 Kelvins (or whatever you decide “cold, dark gas temperature” is), how big would it have to get to initiate fusion just through sheer pressure?
Hi, Aubrey. Good thinking!
However, one may find that your last question is a bit harder to answer than you might have meant. The
flippantshort answer is that fusion can technically happen anywhere in your hypothetical mass of hydrogen at ANY temperature/pressure — and almost certainly DOES, at some point, somewhere, thanks for THAT, quantum mechanics. Despite this, it is highly unlikely to happen anywhere in your cloud, even at stellar pressures/temperatures.Since that’s not very helpful, let me put it another way: the reason that hydrogen fuses in the manner H + H = He is because at some point the two hydrogen nuclei get close enough to each other that the nuclear Strong force overcomes the magnetic forces keeping the positively charged protons apart. This can happen due to quantum effects at most temperatures or pressures (“tunneling” and so forth), but in most cases it happens because they were physically shoved together by kinetic forces. It is exceedingly rare for this to occur at anything below temperatures in the millions of Kelvin AND pressures measured in tera- or peta-Pascals… and isn’t terribly common even then. One notes that even in the core of our Sol, the average H nucleus has to wait BILLIONS of years until the energies focused upon it and its neighbors finally overcome repulsive forces in order to fuse. (*)
So the not-so-flippant answer to your question is that fusion can technically happen at any temperature OR pressure, but you’ll need a ton of BOTH to get things moving reliably. As it happens, if you were to somehow limit your cloud’s temperature to ~3K regardless of circumstance and rely solely on pressure to ignite fusion within? It seems likely (my math is a bit weak here) that it will take such a large amount of matter that the pressures involved will produce degenerate matter (think “neutron star”) or a black hole before you ever get the fusion ball rolling. If one allows things to heat up naturally instead, one can generally expect sustained H + H reactions from a collapsing cloud somewhere in the range of ~12-20 Jupiter-sized masses, as core temperature and pressure reaches ~10 million Kelvin and some 15+ peta Pascals, respectively.
(*) [Nuclear weapons — a.k.a. “fusion bombs” — are actually ignited with fission bombs (smaller, more condensed versions of the Hiroshima bomb), which is why so-called “hydrogen bombs” require a certain amount of U-235 to become reality. These fission devices are the only things that will reliably produce the huge amounts of energy needed to “set off” a consistent fusion reaction under otherwise Earth-normal pressures & temperatures.
As one may imagine, this need for such extreme heat & pressure is also why fusion reactors are so very hard to build, and why “cold” fusion is such an attractive idea — one which has proven, ah, …. shall I say, elusive 😉 … in our attempts to make it a power-generating reality.]
Thanks for the comments, Smokey. You may be right about a pure hydrogen star, but I think a “realistic” gas mix might provide the match this firecracker needs.
In a normal protostar, deuterium burning prevents the core from compressing to the point of hydrogen ignition before the star is done accreting mass. If that didn’t happen, every star would blow away any excess mass once it reached the neighborhood of 2-3 solar masses. At the same time, that deuterium fusion powers convection that keeps fresh fuel flowing to the core, so the proton-proton chain only kicks in once it heats up enough to form a radiative barrier and exhausts its deuterium supply. (I’m not sure how this works in red dwarves that don’t generate a radiative zone… I’ll have to look into that.) So in our hypothetical “cold star”, it may only need to reach enough mass to initiate deuterium fusion, which would then introduce the necessary heat to kick off hydrogen fusion proper.
My back-of-the-envelope calculations say that deuterium fusion requires kinetic energy of 140 keV, which is equivalent to 3.7×10^6 m/s for deuterium (neglecting relativistic effects). That’s just about the same as the surface gravity of a white dwarf, so it might be a a bit of a toss-up whether deuterium fusion started before a degenerate core formed (igniting a normal star) or after (setting off a Type Ia supernova).
Sorry I misspelled your handle earlier!
As I said, my math is a bit weak here, and I certainly did neglect to account for anything other than the P-P variety of fusion on top of that.
It’s an interesting problem, though, isn’t it? I know there are places in space where lots of cold gas clumps together yet remains ‘refrigerated’ at temperatures below what one might otherwise suspect, so it may not be a solely hypothetical situation after all. ^_^
To kill a star? Doesn’t necessarily mean preventing fusion. It means just stopping it from being hospitable. I would just dump a bunch of heavy elements that normally won’t fuse into it. Let it turn into a Neutron Star in density or into a Black Hole. Probably the easiest way to end a star’s nice bright shiny life.