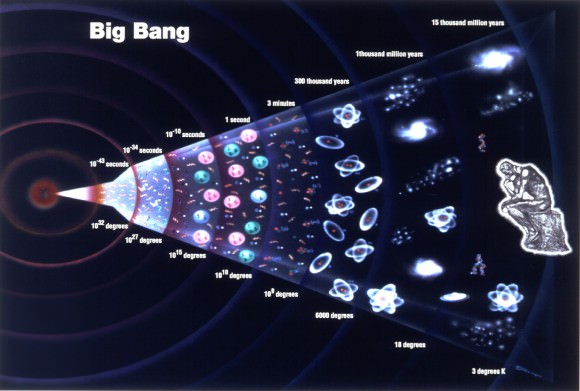
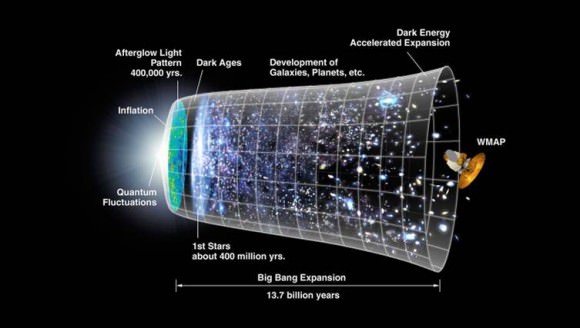
[/caption]
Not long ago, scientists believed that the smallest part of matter was the atom; the indivisible, indestructible, base unit of all things. However, it was not long before scientists began to encounter problems with this model, problems arising out of the study of radiation, the laws of thermodynamics, and electrical charges. All of these problems forced them to reconsider their previous assumptions about the atom being the smallest unit of matter and to postulate that atoms themselves were made up of a variety of particles, each of which had a particular charge, function, or “flavor”. These they began to refer to as Subatomic Particles, which are now believed to be the smallest units of matter, ones that composenucleons and atoms.
Whereas protons, neutrons and electrons have always been considered to be the fundamental particles of an atom, recent discoveries using atomic accelerators have shown that there are actually twelve different kinds of elementary subatomic particles, and that protons and neutrons are actually made up of smaller subatomic particles. These twelve particles are divided into two categories, known as Leptons and Quarks. There are six different kinds, or “flavors”, of quarks (named because of their unusual behavior). These include up, down, charm, strange, top, and bottom quark, each of which possesses a charge that is expressed as a fraction (+2/3 for up, top and charm,-1/3 for down, bottom and strange) and have variable masses. There are also six different types of Leptons, which include Electrons, Muons, Taus, Electron Neutrinos, Muon Neutrinos, and Tau Neutrinos. Whereas electrons and Muons both have a negative charge of -1 (Muons having greater mass), Neutrinos have no charge and are extremely difficult to detect.
In addition to elementary particles, composite particles are another category of subatomic particles. Whereas elementary particles are not made up of other particles, composite particlesare bound states of two or more elementary particles, such as protons or atomic nuclei. For example, a proton is made of two Up quarks and one Down quark, while the atomic nucleus of helium-4 is composed of two protons and two neutrons.In addition, there are also the subatomic particles that fall under the heading of Gauge Bosons, which were identified using the same methods as Leptons and Quarks. These are classified as “force carriers”, i.e. particles that act as carriers for the fundamental forces of nature. These include photons that are associated with electromagnetism, gravitons that are associated with gravity, the three W and Z bosons of weak nuclear forces, and the eight gluons of strong nuclear forces. Scientists also predict the existence of several more, what they refer to as “hypothetical” particles, so the list is expected to grow.
Today, there are literally hundreds of known subatomic particles, most of which were either the result of cosmic rays interacting with matter or particle accelerator experiments.
We have written many articles about the subatomic particles for Universe Today. Here’s an article about the atomic nucleus, and here’s an article about the atomic theory.
If you’d like more info on the Atom, check out the Background on Atoms, and here’s a link to the NASA’s Understanding the Atom Page.
We’ve also recorded an entire episode of Astronomy Cast all about the Composition of the Atom. Listen here, Episode 164: Inside the Atom.
Sources:
http://en.wikipedia.org/wiki/Subatomic_particle
http://en.wikipedia.org/wiki/Nucleon
http://www.school-for-champions.com/science/subatomic.htm
http://en.wikipedia.org/wiki/Gauge_boson
http://en.wikipedia.org/wiki/List_of_particles