Chemistry on other worlds varies widely from that on Earth. Much of Earth’s chemistry is driven by well-understood processes, which typically involve water and heat in some form. Mars lacks both of those features, which makes how some of its chemicals formed a point of ongoing debate in the scientific community. A new paper led by Alian Wang and Neil Sturchio of Washington University of St. Louis and the University of Delaware, respectively, and published recently in Earth and Planetary Science Letters offers a new framework for understanding chemical reaction processes on Mars. Despite the differences, Earthlings will still be familiar with the driving force behind Martian chemistry - electricity.
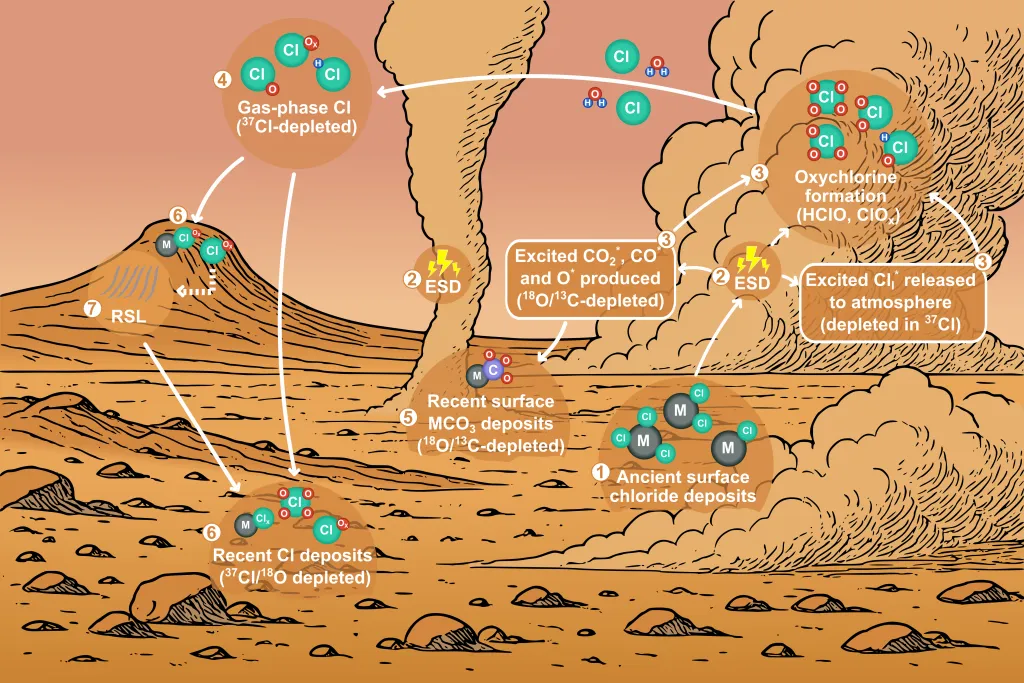
Various rovers and orbits on and around Mars have noticed a peculiar isotopic imbalance on the surface of the Red Planet. An isotopic imbalance can occur when the ratio of two different isotopes of a particular element is skewed from the natural ratios expected. In particular, “heavy” isotopes of some of the most common elements - chlorine, oxygen, and carbon, seem to be lacking on Mars.
For example, Chlorine-37, the “heavy” isotope of that particular element, is 51 parts per thousand (ppt) less abundant than would otherwise be expected on the Martian surface. Given that it is a key ingredient in the hazardous “perchlorates” that stand as one of the major challenges to long-term biological systems living on Mars (like humans), understanding why that imbalance has occurred is key to understanding how we might mitigate the threat those chemicals represent.
Fraser discusses an idea to treat Mars of its toxic perchlorates.The heavy isotope imbalance for carbon (11.4 ppt) and oxygen (22.8 ppt) are less pronounced, but no less important. Both are key ingredients for the formation of carbonates, which previous generations of scientists thought were evidence of previous liquid water on the planet’s surface. So what is causing these imbalances? And what does electricity have to do with it?
Another common feature of Mars’ surface are its famous dust storms. These massive storms take up a significant amount of the planet’s surface in certain seasons. They also form mini-vortices that look like miniaturized tornadoes. Importantly, those storms, and especially those vortices, cause Martian dust they kick up to rub together, eventually building up an electrostatic charge, similar to what happens when you rub a balloon on your head.
But in Mars’ weaker atmosphere, it’s relatively easy to overcome the dielectric constant of the atmosphere itself, allowing small “arcs” that are familiar to anyone who has played under a blanket in a dry room at night. These arcs, known technically as electrostatic discharges, or ESDs, are the driving force of one of the primary chemical cycles on Mars, according to the new paper.
Fraser talks about how a realistic mars mission will play out.The authors built several test chambers, including the Planetary Environment and Analysis Chamber (PEACh), specifically to test how salts commonly found on Mars would react to the electricity produced during a dust storm. They found the ESDs that happen in dust storms create high-energy electrons that run into the CO2 that comprises the Martian atmosphere. When they do so, they create reactive radicals like CO and O. These free radicals then fall to the chloride salts that exist on the ground, bonding oxygen to them, and changing chlorine to perchlorates, the deadly substance carbon-based lifeforms would rather avoid.
But it does explain where they came from. The same process happens for carbonates, which were commonly thought to be formed by liquid water. But like their chlorinated cousins, it seems a wide variety of Mars-based chemicals can form with nothing other than static shocks during a dust storm.
This data matches up much more closely with in-situ and remote observations - in particular the lower density of “heavy” isotopes they’ve found. ESD acts like a “filter”, selecting the lighter atoms to participate in chemical reactions that form the compounds rovers like Curiosity and orbiters like ExoMars have captured. The actual physics behind that process is complicated, but needless to say, this idea that chemical cycles on Mars are driven by dust-derived electrical discharge seems to fit the data very well.
Video of dust devils captured on Mars by Curiosity. These are the types of storms that create ESDs, which then create perchlorates and carbonates on the Martian surface.Such electrical-driven reactions have applications on more than just Mars. Venus, some of the outer Gas Giants, and even our own Moon, could have their own version of ESD-driven chemical reaction chains. While that means there’s plenty more to study, there’s also a cautionary tale for future Martian explorers. ESD is an ongoing, active process that is part of Mars’ natural, cold climate. That means that perchlorates, the deadly chemicals that might very well hinder our tentative efforts at a permanent base on the Red Planet, are constantly being created there.
While that’s certainly not a deal breaker for exploration, it is something we need to be aware of. But the authors certainly aren’t done with their exploration of the impact that small arcs of electricity have on driving chemical reactions throughout the solar system. Expect more papers about how arcs on Venus affect that planet’s surface chemistry soon.
Learn More:
WUSL - The Electrifying Science Behind Martian Dust
N. C. Sturchio et al. - Isotope effects (Cl, O, C) of heterogeneous electrochemistry induced by Martian dust activities
UT - Toxic Perchlorate on Mars Could Make Life More Interesting
 Universe Today
Universe Today