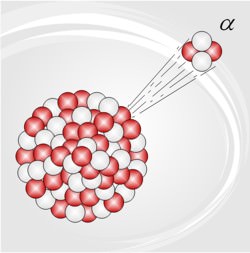
When a nucleus undergoes radioactive decay – or decays, radioactively – it changes its state to one of a lower energy, and emits a particle (sometimes more than one), a gamma ray, or both (and one type of radioactive decay involves the absorption, or capture, of an electron as well emission of a particle).
Among radioactive materials which occur naturally here on Earth, two kinds of radioactive decay are common: alpha (α) and beta (β). They get their names from the most obvious particles emitted, an alpha particle (which is the nucleus of the stable isotope of helium called helium-4) or a beta particle (which is either an electron or a positron; the positron is the antimatter counterpart to the electron). In either kind of decay a photon with gamma ray energy may be emitted too, and in beta decay a neutrino is nearly always emitted (antineutrino if it’s electron-type beta decay, neutrino if positron-type).
In the lab, and out in space, there are atomic nuclei which undergo radioactive decay in other ways – by emitting a proton, for example; these types of decay occur in isotopes which have very short lives.
You’ve heard of Schrödinger’s poor cat, right? Well, not so poor, because it’s a thought experiment (no real cat involved), but it’s a good device for understanding something rather quirky about radioactive decay. You see, if you have a few billion atoms of a radioactive isotope, potassium-40 say, you can say with great certainty how many will decay in the next year. However, you cannot say which particular nuclei will decay!
Radioactive decay is very important for a wide range of human activities, from medicine to electricity production and beyond, and also to astronomers. For example, the characteristic light curve of Type Ia supernovae – which are used to estimate the age of the universe (among other things) – comes from the decay of a radioactive isotope of nickel (nickel-56, and its daughter isotope, cobalt-56), produced in copious quantities by the suicidal star.
There’s a lot of material, out there on the web, on radioactive decay; here are some good links for you to click on: Radioactive Decay in Supernova Remnants (NASA), Radioactive Decay (Carlton College), and Decay (an applet, Michigan State University).
Universe Today stories and articles on radioactive decay include Solved: Mystery of Gamma Ray Distribution in the Milky Way, A Supernova Every 50 Years, and Add Heat, Then Tectonics: Narrowing the Hunt for Life in Space.
Astronomy Cast episodes of direct relevance to radioactive decay include The Strong and Weak Nuclear Forces and Energy Levels and Spectra; check them out!
Sources:
Wikipedia
Boston University
NDT Resource Center
Stanford University