[/caption]
It was just over a century ago that a little known French scientist named Henri Becquerel came across something new and immensely startling. At the time, while working with phosphorescent materials (i.e. materials that glow in the dark after being subjected to light), he discovered naturally occurring rays that he couldn’t account for. In time, these rays were discovered to be present in several naturally occurring elements, and were dubbed radioactivity. Those metals that exhibited them also came to be known as Radioactive Isotopes.
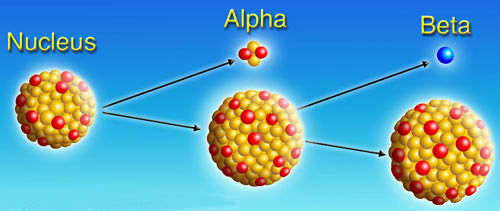
Radioisotopes, (also known as radioactive isotopes or radionuclides), are atoms with a different number of neutrons than a usual atom. Due to this imbalance, these isotopes have an unstable nucleus that decays, and in the process emitting alpha, beta and gamma rays until the isotope reaches stability. Once it’s stable, the isotope has transformed into another element entirely. Every chemical element has one or more radioisotopes, with over 1,000 isotopes accounted for in total. Approximately 50 of these are found in nature; the rest are produced artificially as the direct result of nuclear reactions or indirectly as the radioactive descendants of these products.
Of the naturally occurring radioisotopes, there are three categories that are used to group them. The first is primordial radionuclides, which originate mainly within the interior of stars and like uranium and thorium, are still present because their half-lives are so long that they have not yet completely decayed. The second group, secondary radionuclides, are radiogenic isotopes derived from the decay of primordial radionuclides and are characterized by their shorter half-lives. The third and final group is known cosmogenic radionuclides, which consists of isotopes like Carbon 14 which are constantly produced in the atmosphere due to cosmic rays. Artificially produced radionuclides, on the other hand, are produced by nuclear reactors, particle accelerators or by radionuclide generators (where a parent isotope, usually produced in a nuclear reactor, is allowed to decay to produce a radioisotope). In addition, nuclear explosions are known to produce artificial radioisotopes as well.
Radioisotopes are used today for a variety of purposes. When it comes to the field of nuclear medicine, radioactive isotopes are used in MRI’s and X-rays for diagnostic purposes, for targeted radiation therapy, and to sterilize medical equipment. In biochemistry and genetics, radionuclides are used in molecular and DNA research in order to “label” molecules and trace chemical and physiological processes. Carbon-14, a naturally occurring cosmogenic isotope, is used for carbon dating by archeologists, paleontologists, and geologists. In agriculture, radiation is used to stop the sprouting of root crops, kill parasites and pests, and in veterinary medicine. And when it comes to industry, radionuclides are used to study the rate of wear and corrosion of metals, to test for leaks and seams, analyze pollutants, study the movement of surface water, measure water runoffs from rain and snow, and the flow rates of streams and rivers.
We have written many articles about radioisotopes for Universe Today. Here’s an article about isotopes, and here’s an article about radioactive decay.
If you’d like more info on radioisotopes, check out these articles from NDT Resource Center and Science Courseware.
We’ve also recorded an entire episode of Astronomy Cast all about the Age of the Universe. Listen here, Episode 122: How Old is the Universe?.
References:
http://en.wikipedia.org/wiki/Radionuclide
http://en.wikipedia.org/wiki/Radioactive_decay
http://www.britannica.com/EBchecked/topic/489027/radioactive-isotope
http://en.wikipedia.org/wiki/Radiocarbon_dating
http://www.ehow.com/about_5095610_radioactive-isotopes.html