Ever since it was first proposed by Democritus in the 5th century BCE, the atomic model has gone through several refinements over the past few thousand years. From its humble beginnings as an inert, indivisible solid that interacts mechanically with other atoms, ongoing research and improved methods have led scientists to conclude that atoms are actually composed of even smaller particles that interact with each other electromagnetically.
This was the basis of the atomic theory devised by English physicist J.J. Thompson in the late 19th an early 20th centuries. As part of the revolution that was taking place at the time, Thompson proposed a model of the atom that consisted of more than one fundamental unit. Based on its appearance, which consisted of a “sea of uniform positive charge” with electrons distributed throughout, Thompson’s model came to be nicknamed the “Plum Pudding Model”.
Though defunct by modern standards, the Plum Pudding Model represents an important step in the development of atomic theory. Not only did it incorporate new discoveries, such as the existence of the electron, it also introduced the notion of the atom as a non-inert, divisible mass. Henceforth, scientists would understand that atoms were themselves composed of smaller units of matter and that all atoms interacted with each other through many different forces.
Atomic Theory to the 19th century:
The earliest known examples of atomic theory come from ancient Greece and India, where philosophers such as Democritus postulated that all matter was composed of tiny, indivisible and indestructible units. The term “atom” was coined in ancient Greece and gave rise to the school of thought known as “atomism”. However, this theory was more of a philosophical concept than a scientific one.
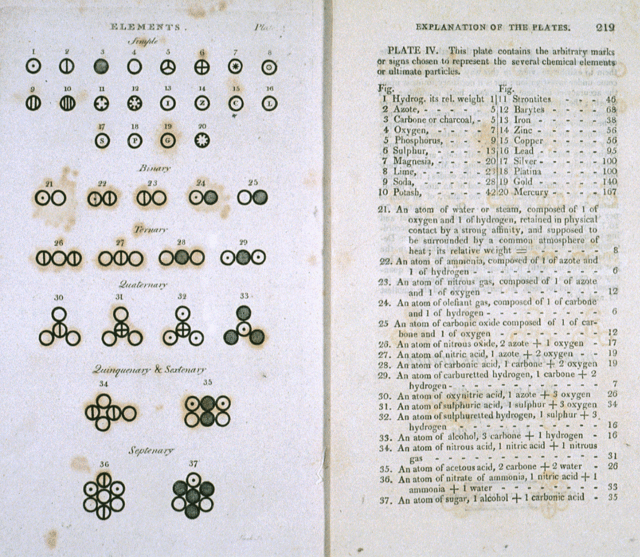
It was not until the 19th century that the theory of atoms became articulated as a scientific matter, with the first evidence-based experiments being conducted. For example, in the early 1800s, English scientist John Dalton used the concept of the atom to explain why chemical elements reacted in certain observable and predictable ways.
Dalton began with the question of why elements reacted in ratios of small whole numbers and concluded that these reactions occurred in whole-number multiples of discrete units – i.e. atoms. Through a series of experiments involving gases, Dalton went on to develop what is known as Dalton’s Atomic Theory. This theory expanded on the laws of conversation of mass and definite proportions – formulated by the end of the 18th century – and remains one of the cornerstones of modern physics and chemistry.
The theory comes down to five premises: elements, in their purest state, consist of particles called atoms; atoms of a specific element are all the same, down to the very last atom; atoms of different elements can be told apart by their atomic weights; atoms of elements unite to form chemical compounds; atoms can neither be created or destroyed in chemical reaction, only the grouping ever changes.
By the late 19th century, scientists also began to theorize that the atom was made up of more than one fundamental unit. However, most scientists ventured that this unit would be the size of the smallest known atom – hydrogen. By the end of the 19th century, the situation would change drastically.
Thompson’s Experiments:
Sir Joseph John Thomson (aka. J.J. Thompson) was an English physicist and the Cavendish Professor of Physics at the University of Cambridge from 1884 onwards. During the 1880s and 1890s, his work largely revolved around developing mathematical models for chemical processes, the transformation of energy in mathematical and theoretical terms, and electromagnetism.
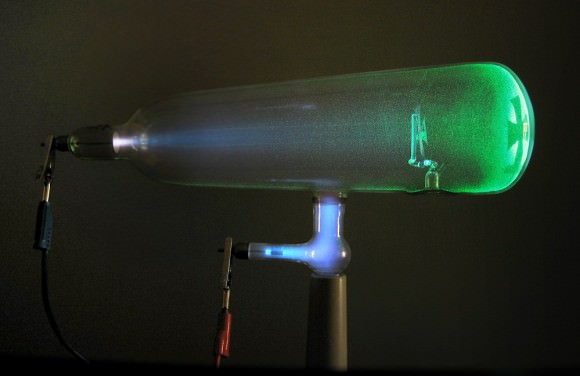
However, by the late 1890s, he began conducting experiments using a cathode ray tube known as the Crookes’ Tube. This consists of a sealed glass container with two electrodes that are separated by a vacuum. When voltage is applied across the electrodes, cathode rays are generated (which take the form of a glowing patch of gas that stretches to the far end of the tube).
Through experimentation, Thomson observed that these rays could be deflected by electric and magnetic fields. He concluded that rather than being composed of light, they were made up of negatively charged particles he called “corpuscles”. Upon measuring the mass-to-charge ration of these particles, he discovered that they were 1ooo times smaller and 1800 times lighter than hydrogen.
This effectively disproved the notion that the hydrogen atom was the smallest unit of matter, and Thompson went further to suggest that atoms were divisible. To explain the overall charge of the atom, which consisted of both positive and negative charges, Thompson proposed a model whereby the negatively charged corpuscles were distributed in a uniform sea of positive charge.

These corpuscles would later be named “electrons”, based on the theoretical particle predicted by Anglo-Irish physicist George Johnstone Stoney in 1874. And from this, the Plum Pudding Model was born, so named because it closely resembled the English desert that consists of plum cake and raisins. The concept was introduced to the world in the March 1904 edition of the UK’s Philosophical Magazine, to wide acclaim.
Problems With the Plum Pudding Model:
Unfortunately, subsequent experiments revealed a number of scientific problems with the model. For starters, there was the problem of demonstrating that the atom possessed a uniform positive background charge, which came to be known as the “Thomson Problem”. Five years later, the model would be disproved by Hans Geiger and Ernest Marsden, who conducted a series of experiments using alpha particles and gold foil.
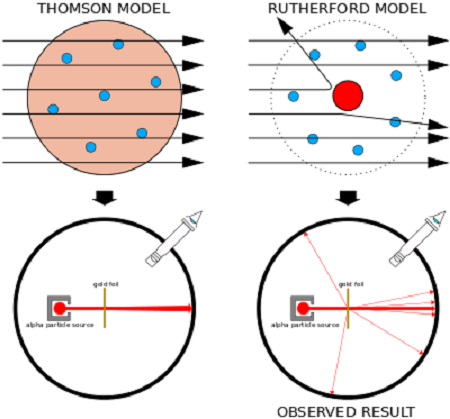
In what would come to be known as the “gold foil experiment“, they measured the scattering pattern of the alpha particles with a fluorescent screen. If Thomson’s model were correct, the alpha particles would pass through the atomic structure of the foil unimpeded. However, they noted instead that while most shot straight through, some of them were scattered in various directions, with some going back in the direction of the source.
Geiger and Marsden concluded that the particles had encountered an electrostatic force far greater than that allowed for by Thomson’s model. Since alpha particles are just helium nuclei (which are positively charged) this implied that the positive charge in the atom was not widely dispersed, but concentrated in a tiny volume. In addition, the fact that those particles that were not deflected passed through unimpeded meant that these positive spaces were separated by vast gulfs of empty space.
.
By 1911, physicist Ernest Rutherford interpreted the Geiger-Marsden experiments and rejected Thomson’s model of the atom. Instead, he proposed a model where the atom consisted of mostly empty space, with all its positive charge concentrated in its center in a very tiny volume, that was surrounded by a cloud of electrons. This came to be known as the Rutherford Model of the atom.
Subsequent experiments by Antonius Van den Broek and Neils Bohr refined the model further. While Van den Broek suggested that the atomic number of an element is very similar to its nuclear charge, the latter proposed a Solar-System-like model of the atom, where a nucleus contains the atomic number of positive charge and is surrounded by an equal number of electrons in orbital shells (aka. the Bohr Model).
Though it would come to be discredited in just five years time, Thomson’s “Plum Pudding Model” would prove to be a crucial step in the development of the Standard Model of particle physics. His work in determining that atom’s were divisible, as well as the existence of electromagnetic forces within the atom, would also prove to be major influence on the field of quantum physics.
We have written many interesting articles on the subject of atomic theory here at Universe Today. For instance, here is How Many Atoms Are There In The Universe?, John Dalton’s Atomic Model, What Are The Parts Of The Atom?, Bohr’s Atomic Model,
For more information, be sure to check out Physic’s Worlds pages on 100 years of the electron: from discovery to application and Proton and neutron masses calculated from first principles
Astronomy Cast also has some episodes on the subject: Episode 138: Quantum Mechanics, Episode 139: Energy Levels and Spectra, Episode 378: Rutherford and Atoms and Episode 392: The Standard Model – Intro.


