[/caption]
For most people, the words “ideal gas” might conjure up the image of some kind of super fuel, perhaps a near-inexhaustible kind that creates zero air pollution! Sadly, this is not what is meant by ideal gas. In reality, an ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. At normal conditions such as standard temperature and pressure, most real gases such as air, nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide behave like an ideal gas and can be treated as such within reasonable tolerances. It is only when they are treated with higher temperatures and lower pressure that they deviate from this trend. Once they get into this territory, experimental gas laws, such as Charles’s Law, come into play.
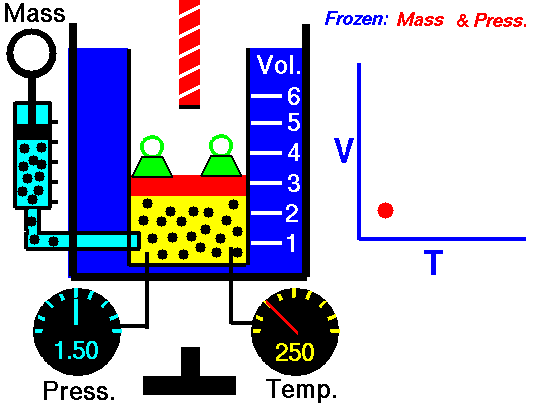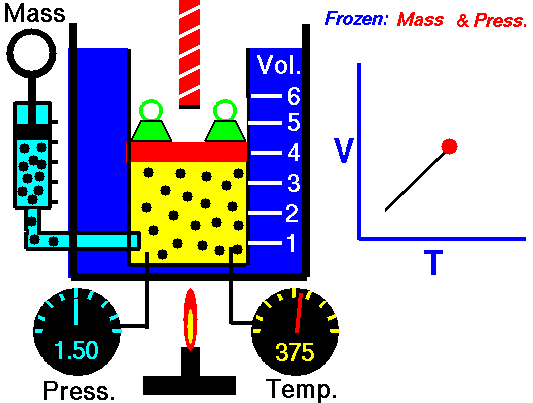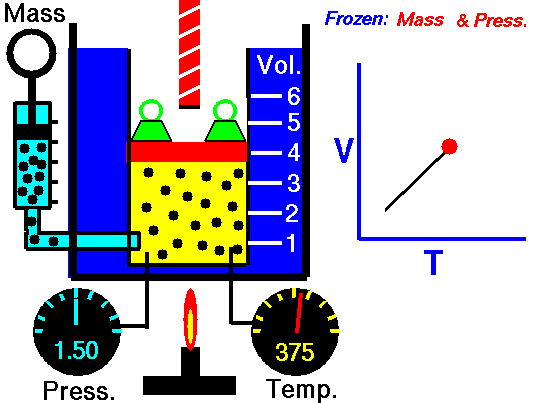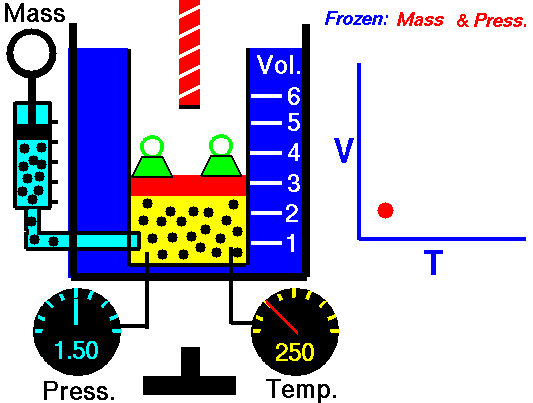
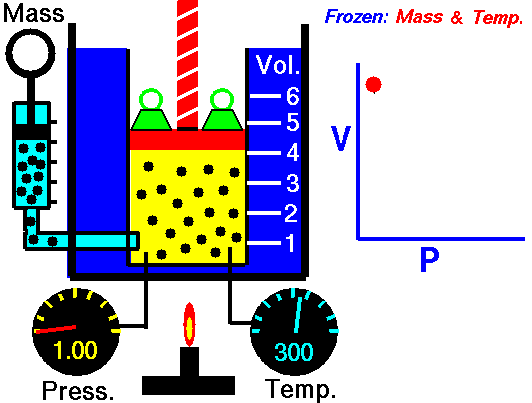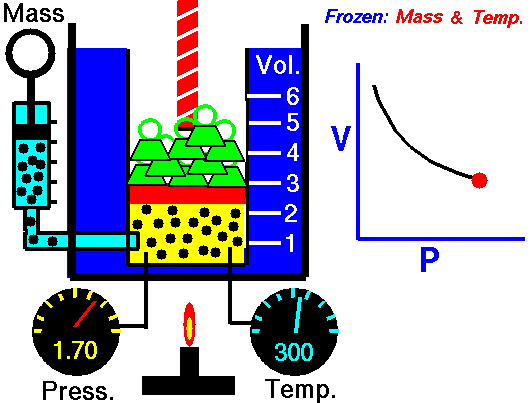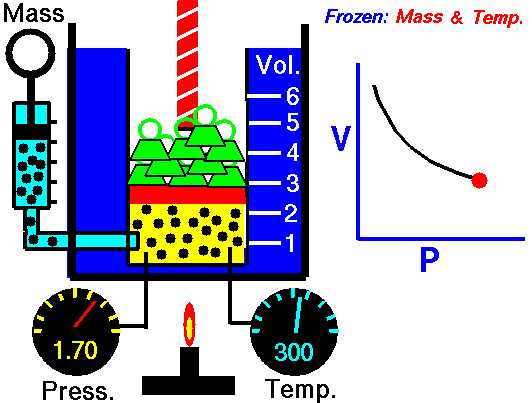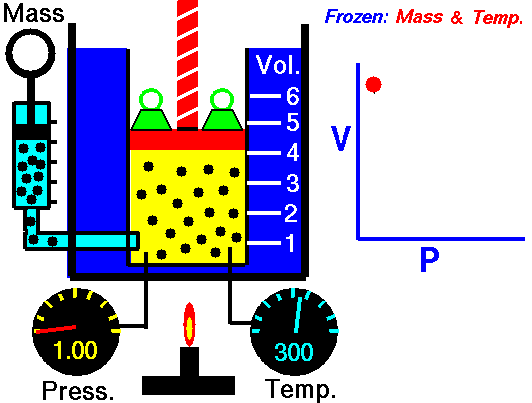
Also known as the law of volumes, Charles’s Law is an experimental gas law which describes how gases tend to expand when heated. It was first published by French natural philosopher Joseph Louis Gay-Lussac in 1802, although he credited the discovery to unpublished work from the 1780s by Jacques Charles, hence the name. This law applies generally to all gases, and also to the vapours of volatile liquids if the temperature is more than a few degrees above the boiling point. Given the interest in hot air balloons at the time, it is certainly understandable why Gay-Lussac, Charles and other scientists around the globe were so interested in the relationship between volume, pressure and temperature when it came to gasses.
In lay terms, the law states that: at constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. the gas expands as the temperature increases). This can be written as: V? T, where V is the volume of the gas; and T is the absolute temperature. In mathematical terms, the law can also be expressed as: V100 – V0 = kV0, where V100 is the volume occupied by a given sample of gas at 100 °C; V0 is the volume occupied by the same sample of gas at 0 °C; and k is a constant which is the same for all gases at constant pressure. Gay-Lussac’s value for k was ½.6666, remarkably close to the present-day value of ½.7315.
Combined with Boyle’s law, these laws make up what is known as the “Ideal Gas Law” which was first stated by ÉmileClapeyron in 1834.
We have written many articles about Charles’s Law for Universe Today. Here’s an article about the Combined Gas Law, and here’s an article about Boyle’s Law.
If you’d like more info on Charles’s Law, check out a discussion about Charles’s Law, and here’s a link to an article about Charles’s Law by the Glenn Research Center.
We’ve also recorded an episode of Astronomy Cast all about planet Earth. Listen here, Episode 51: Earth.
Sources:
http://en.wikipedia.org/wiki/Charles%27s_law
http://en.wikipedia.org/wiki/Ideal_gas
http://www.chm.davidson.edu/vce/gaslaws/charleslaw.html
http://www.grc.nasa.gov/WWW/K-12/airplane/glussac.html
http://en.wikipedia.org/wiki/Ideal_gas_law