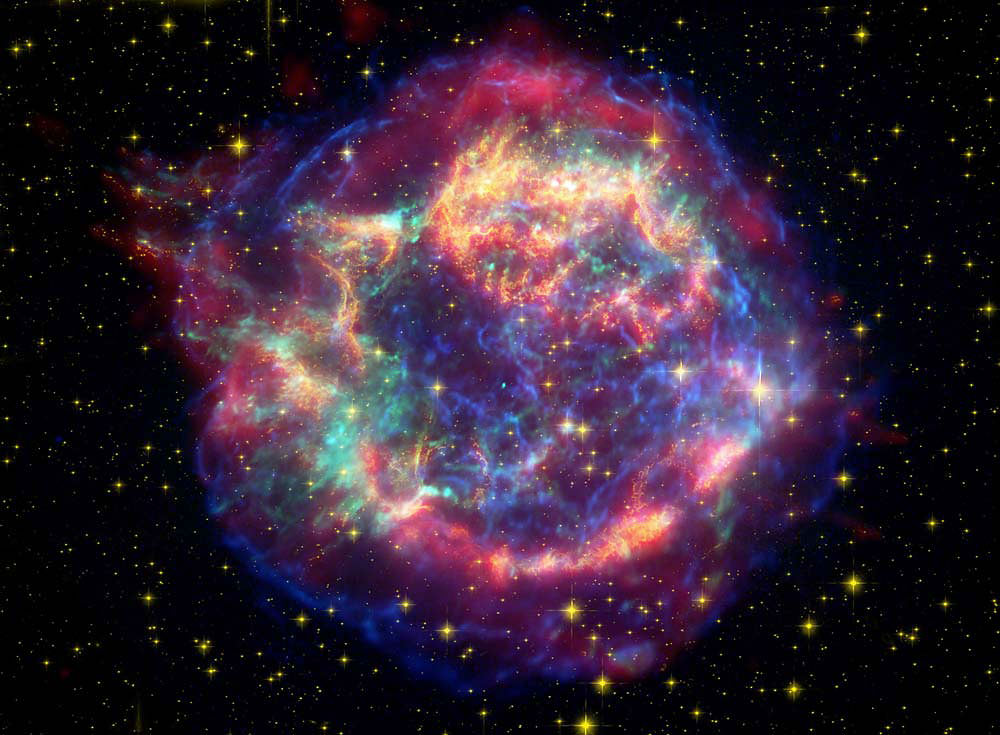
“The total number of stars in the Universe is larger than all the grains of sand on all the beaches of the planet Earth,” Carl Sagan famously said in his iconic TV series Cosmos. But when two of those grains are made of a silicon-and-oxygen compound called silica, and they were found hiding deep inside ancient meteorites recovered from Antarctica, they very well may be from a star… possibly even the one whose explosive collapse sparked the formation of the Solar System itself.
Researchers from Washington University in St. Louis with support from the McDonnell Center for the Space Sciences have announced the discovery of two microscopic grains of silica in primitive meteorites originating from two different sources. This discovery is surprising because silica — one of the main components of sand on Earth today — is not one of the minerals thought to have formed within the Sun’s early circumstellar disk of material.
Instead, it’s thought that the two silica grains were created by a single supernova that seeded the early solar system with its cast-off material and helped set into motion the eventual formation of the planets.
According to a news release by Washington University, “it’s a bit like learning the secrets of the family that lived in your house in the 1800s by examining dust particles they left behind in cracks in the floorboards.”

Until the 1960s most scientists believed the early Solar System got so hot that presolar material could not have survived. But in 1987 scientists at the University of Chicago discovered miniscule diamonds in a primitive meteorite (ones that had not been heated and reworked). Since then they’ve found grains of more than ten other minerals in primitive meteorites.
The scientists can tell these grains came from ancient stars because they have highly unusual isotopic signatures, and different stars produce different proportions of isotopes.
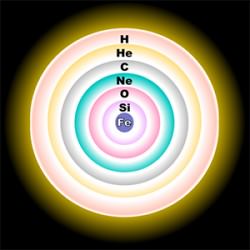
But the material from which our Solar System was fashioned was mixed and homogenized before the planets formed. So all of the planets and the Sun have the pretty much the same “solar” isotopic composition.
Meteorites, most of which are pieces of asteroids, have the solar composition as well, but trapped deep within the primitive ones are pure samples of stars, and the isotopic compositions of these presolar grains can provide clues to their complex nuclear and convective processes.
Some models of stellar evolution predict that silica could condense in the cooler outer atmospheres of stars, but others say silicon would be completely consumed by the formation of magnesium- or iron-rich silicates, leaving none to form silica.
“We didn’t know which model was right and which was not, because the models had so many parameters,” said Pierre Haenecour, a graduate student in Earth and Planetary Sciences at Washington University and the first author on a paper to be published in the May 1 issue of Astrophysical Journal Letters.
Under the guidance of physics professor Dr. Christine Floss, who found some of the first silica grains in a meteorite in 2009, Haenecour investigated slices of a primitive meteorite brought back from Antarctica and located a single grain of silica out of 138 presolar grains. The grain he found was rich in oxygen-18, signifying its source as from a core-collapse supernova.
Finding that along with another oxygen-18-enriched silica grain identified within another meteorite by graduate student Xuchao Zhao, Haenecour and his team set about figuring out how such silica grains could form within the collapsing layers of a dying star. They found they could reproduce the oxygen-18 enrichment of the two grains through the mixing of small amounts of material from a star’s oxygen-rich inner zones and the oxygen-18-rich helium/carbon zone with large amounts of material from the outer hydrogen envelope of the supernova.
In fact, Haenecour said, the mixing that produced the composition of the two grains was so similar, the grains might well have come from the same supernova — possibly the very same one that sparked the collapse of the molecular cloud that formed our Solar System.
“It’s a bit like learning the secrets of the family that lived in your house in the 1800s by examining dust particles they left behind in cracks in the floorboards.”
Ancient meteorites, a few microscopic grains of stellar sand, and a lot of lab work… it’s an example of cosmic forensics at its best!