The southern portion of Mercury’s Vivaldi basin and outlying rugged terrain
Named for the 17th-century Venetian composer, the southern half of Mercury’s Vivaldi basin is seen in this image acquired on August 26 by NASA’s MESSENGER spacecraft. The 213-km (132-mile) -wide crater’s smooth floor is contrasted by the incredibly rugged terrain beyond its outermost ring — a result of the ejected material that was flung out from the impact site and emphasized by the low angle of illumination.
The floor of the crater remained relatively smooth due to molten material that erupted in the wake of the impact event, flooding the basin.
Recent findings from the MESSENGER mission have revealed variations in Mercury’s surface composition due to volcanism that occurred at different times, as well as a surprising concentration of elements like magnesium and sulfur — much more so than any of the other terrestrial planets.
In results to be published in the Journal of Geophysical Research, scientists report that Mercury’s volcanic smooth plains differ in composition from older surrounding terrains. The older terrain has higher ratios of magnesium to silicon, sulfur to silicon, and calcium to silicon, but lower ratios of aluminum to silicon, suggesting that the smooth plains material erupted from a magma source that was chemically different from the source of the material in the older regions, according to Shoshana Weider of the Carnegie Institution of Washington, the lead author on the paper.
Mercury’s surface was also found to be high in magnesium and sulfur-enriched minerals.
 “None of the other terrestrial planets have such high levels of sulfur. We are seeing about ten times the amount of sulfur than on Earth and Mars,” Weider said. “In terms of magnesium, we do have some materials on Earth that are high in magnesium. They tend to be ancient volcanic rocks that formed from very hot lavas. So this composition on Mercury tells us that eruptions of high-temperature lavas might have formed these high-magnesium materials.”
“None of the other terrestrial planets have such high levels of sulfur. We are seeing about ten times the amount of sulfur than on Earth and Mars,” Weider said. “In terms of magnesium, we do have some materials on Earth that are high in magnesium. They tend to be ancient volcanic rocks that formed from very hot lavas. So this composition on Mercury tells us that eruptions of high-temperature lavas might have formed these high-magnesium materials.”
Read: MESSENGER Reveals Mercury’s Colors
The data was gathered with MESSENGER’s X-Ray Spectrometer (XRS) — one of two instruments designed to measure the abundances of many key elements in the top 2mm of Mercury’s crust. XRS detects emissions from elements in the 1-10 kiloelectron-volt (keV) range – specifically, magnesium, aluminum, silicon, sulfur, calcium, titanium, and iron.
Read more on the MESSENGER mission site here.
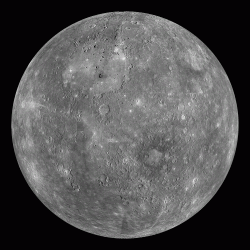
Inset image: A global mosaic of Mercury from MESSENGER (2011). Image credits: NASA/Johns Hopkins University Applied Physics Laboratory/Carnegie Institution of Washington