Canadians don’t have much to be proud of, but we can regale you with our ability to withstand freezing cold temperatures. Now, I live on the West Coast, so I’m soft and weak, rarely experiencing temperatures below freezing.
But for some of my Canadian brethren, temperatures can dip down to levels your mind and body can scarcely comprehend. For example, I have a friend who lives in Winnipeg, Manitoba. For a day last winter, the temperatures there dipped down -31C, but with the windchill, it felt like -50C. On that same day, it was a balmy -29C on Mars. On Mars!
But for scientists, and the Universe, it can get much much colder. So cold, in fact, that they use a completely different temperature scale – Kelvin – to measure how far away things are from the coldest possible temperature: Absolute Zero.

On the Celsius scale, Absolute Zero is -273.15 degrees. And in Fahrenheit, it’s -459.67 degrees. In the Kelvin scale, however, it’s very simple. Absolute Zero is 0 kelvin.
At this point, a science explainer is going to stumble into a minefield of incorrect usage. It’s not 0 degrees kelvin, you don’t say the degrees part, just the kelvin part. Just kelvin.
This is because when you measure something from an arbitrary point, like the direction you just turned, you’ve changed course 15-degrees. But if you’re measuring from an absolute point, like the lowest physical temperature defined by nature, you drop the degrees because it’s an absolute. An Absolute Zero.
Of course, I’ve probably gotten that wrong too. This stuff is hard.
Anyway, back to Absolute Zero.

Absolute Zero is the coldest possible temperature that can theoretically be reached. At this point, no heat energy can be extracted from a system, no work can be done. It’s dead Jim.
But it’s completely theoretical. It’s practically impossible to cool something down to Absolute Zero. In order to cool something down, you need to do work to extract heat from it. The colder you get, the more work you need to do. In order to get to Absolute Zero, you’d need to put in an infinite amount of work. And that’s ridiculous.
As you probably learned in physics or chemistry class, the temperature of a gas translates to the motion of the particles in the gas. As you cool a gas down, by extracting heat from it, the particles slow down.
You would think, then, that by cooling something down to Absolute Zero, all particle motion in that something would stop. But that’s not true.
From a quantum mechanics point of view, you can never know the position and momentum of particles at the same time. If the particles stopped, you’d know their momentum (zero) and their position… right there. The Universe and its laws of physics just can’t allow that to happen. Thank Heisenberg’s Uncertainty Principle.
Therefore, there’s always a little motion, even if you could get to Absolute Zero, which you can’t. But you can’t extract any more heat from it.
The physicist Robert Boyle was one of the first to consider the possibility that there was a lowest possible temperature, which he called the primum frigidum. In 1702, Guillaume Amontons created a thermometer that he calculated would bottom out at -240 C. Pretty close, actually.
But it was Lord Kelvin, who created this absolute scale in 1848, starting at -273 C, or 0 kelvin.

By this measurement, even with its windchill, Winnipeg was a balmy 223 kelvin on that wintry day.
The surface of Pluto, on the other hand varies from a low of 33 kelvin to a high of 55 kelvin. That’s -240 C to -218 C.
The average background temperature across the entire Universe is just 2.7 kelvin. You won’t find many places that cold, unless you get out to the vast cosmic voids that separate galaxy clusters.
Over time, the background temperature of the Universe will continue to drop, but it’ll never actually reach Absolute Zero. Even in a Googol years, when the last supermassive black hole has finally evaporated, and there’s no usable heat left in the entire Universe.
In fact, astronomers call this bleak future the “heat death” of the Universe. It’s heat death, as in, the death of all heat. And happiness.
You might be surprised to know that the coldest temperature in the entire Universe is right here on Earth. Well, sometimes, anyway. And assuming the aliens haven’t got better technology than us, which they probably do.
At the time that I’m recording this video, physicists have used lasers to cool down Rubidium-87 gas to just 170 nanokelvin, a tiny fraction above Absolute Zero. In fact, they won a Nobel Prize for their work in discovering Bose-Einstein condensates.
NASA is actually working on a new experiment called the Cold Atom Lab that will send a version of this technology to the International Space Station, where it should be able to cool material down to 100 picokelvin. That’s cold.
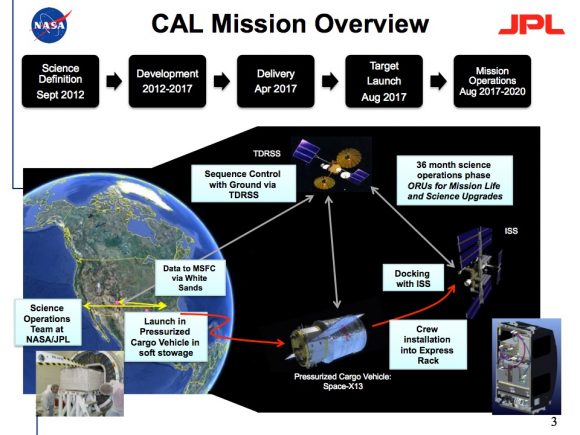
Here are your takeaways. Absolute Zero is the coldest possible temperature than can ever be reached, the point at which no further heat energy can be extracted from a system. Never say degrees kelvin, you’ll cause so much wincing. The Universe can’t match our cold generating abilities… yet. Take that Universe.
I’d love to hear the coldest temperature you’ve ever personally experienced. For me, it was visiting Buffalo in December. That’s not right.




