Scientists are still trying to understand the origin of multicellular life. It emerged about 1.2 billion years ago, or even earlier, according to some debated evidence. The timing of multicellular life's appearance on Earth is not the only thing being debated; so are the mechanisms behind it. New research supports the idea that multicellular life began when single-celled bacteria began grouping together.
The Colonial Theory says that single cells of the same type started living in close proximity to one another and gained evolutionary advantages by doing so. Over time, individual cells adhered to one another and became permanently bound together. Then, the individual cells started exchanging chemical signals, and cells began to take on specialized functions. Eventually, the individual cells couldn't survive on their own and became completely interdependent.
Scientists know of only one type of single-celled bacteria without a unicellular stage that survives by grouping together like multicellular organisms. They're called 'multicellular magnetotactic bacteria,' or MMB. Magnetotactic means they use Earth's magnetic field to move around and orient themselves. They're also considered to exhibit obligate multicellularity, which means that they can't survive alone.
MMB are difficult to study because they resist cultivation in the laboratory. Most of what scientists know about them comes from observation. In research published in PLOS Biology, NASA-funded research examined MMB on a genetic level and learned some surprising things. It appears that MMB are more complex than thought.
The research is titled "Multicellular magnetotactic bacteria are genetically heterogeneous consortia with metabolically differentiated cells." The lead author is George Schaible from the Department of Chemistry and Biochemistry at Montana State University.
Consortia of multicellular magnetotactic bacteria (MMB) are currently the only known example of bacteria without a unicellular stage in their life cycle," the researchers write. "To study the biology of these unique organisms in more detail, we use multiple culture-independent approaches to analyze the genomics and physiology of MMB consortia at single-cell resolution.
The research shows that MMB cells are not identical. Instead, individual cells have slightly different genetic blueprints. This sets them apart from other bacteria that form into aggregates of single cells. For example, colonies of cyanobacteria form stromatolites. The difference is that cyanobacteria can survive alone while MMBs can't.
We separately sequenced the metagenomes of 22 individual MMB consortia, representing 8 new species, and quantified the genetic diversity within each MMB consortium," the researchers write. "This revealed that, counter to conventional views, cells within MMB consortia are not clonal.
The researchers found that individual cells within MMB consortia perform different functions. These different roles contribute to group survival. Even though they're not combined into a single organism, this is very similar to how multicellular organisms like us function. This research shows that much like how humans have fat cells, bone marrow cells, and other types of cells with specialized functions, MMB consortia have functional complexity among their different cells.
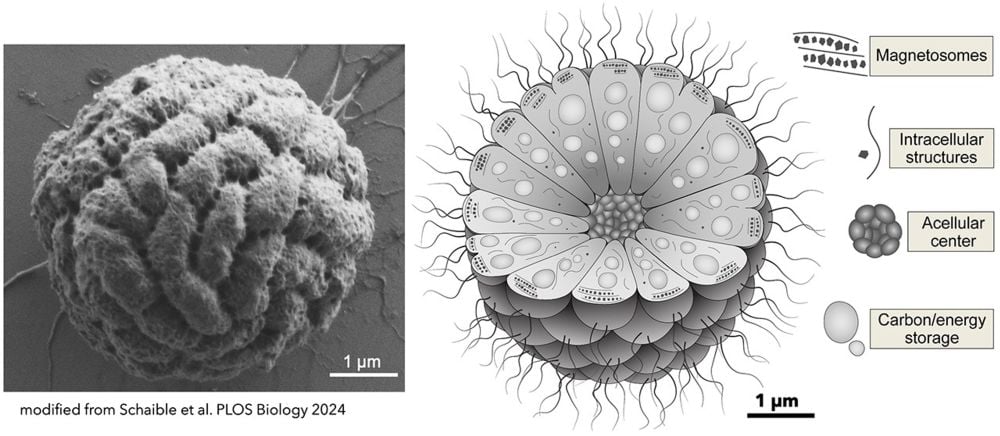 *This image and schematic show the detail in MMB. Individual cells are organized around an acellular center. Each cell contains a magnetosome, an intracellular organelle that holds iron inside a lipid membrane. The acellular center is material devoid of cells but still containing the extracellular matrix. Each cell has a compartment for energy and carbon storage. Intracellular structures in MMB are currently unidentified. Image Credit: George Schaible et al. PLOS Biology 2024*
*This image and schematic show the detail in MMB. Individual cells are organized around an acellular center. Each cell contains a magnetosome, an intracellular organelle that holds iron inside a lipid membrane. The acellular center is material devoid of cells but still containing the extracellular matrix. Each cell has a compartment for energy and carbon storage. Intracellular structures in MMB are currently unidentified. Image Credit: George Schaible et al. PLOS Biology 2024*
"We demonstrate that MMB consortia are mixotrophic sulphate reducers and that they exhibit metabolic differentiation between individual cells, suggesting that MMB consortia are more complex than previously thought," the researchers explain.
This research has broader implications for our understanding of multicellular life. We know that multicellularity evolved in different lineages and led to plants, animals, and fungi. Scientists acknowledge three overall phases in that evolution: cell-to-cell adhesion, cell-to-cell communication, cooperation, specialization, and, at least in some cases, a transition from simple multicellularity to more complex multicellularity.
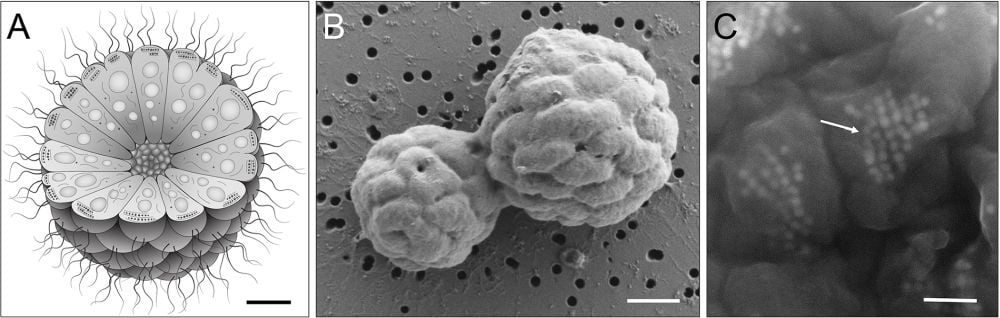 This image shows an MMB inA.Bshows two MMB possibly dividing.C*shows magnetosome chains within individual cells. Image Credit: George Schaible et al. PLOS Biology 2024*
This image shows an MMB inA.Bshows two MMB possibly dividing.C*shows magnetosome chains within individual cells. Image Credit: George Schaible et al. PLOS Biology 2024*
Once multicellular life appeared, Earth was never the same. Its appearance triggered a cascade of effects. Not only did new lifeforms arise, but so did new ecological niches and food webs. It changed Earth's atmosphere, carbon cycle, and biogeochemistry and drove evolution.
Scientists don't all agree on whether MMB are truly multicellular organisms. There's a lot of nuance. Most biologists describe them as 'simple' or 'primitive' multicellularity. In that sense, they represent an intermediate step between single-celled organisms and truly multicellular organisms.
This research sheds some light on how multicellularity may have emerged. MMB are the only bacteria with obligate multicellularity, meaning they can't survive or reproduce independently. They could be showing us how life on Earth took the profound step from unicellularity to multicellularity.
More:
 Universe Today
Universe Today