We have comets and asteroids to thank for Earth's water, according to the most widely-held theory among scientists. But it's not that cut-and-dried. It's still a bit of a mystery, and a new study suggests that not all of Earth's water was delivered to our planet that way.
Hydrogen is the most abundant element in the universe, and it's at the center of the question surrounding Earth's water. This new study was co-led by Peter Buseck, Regents' Professor in the School of Earth and Space Exploration and School of Molecular Sciences at Arizona State University. In it, the authors suggest that the hydrogen came, at least partially, from the
solar nebula
, a cloud of gas and dust left over after the Sun formed.
Before we dig in to the details in this new study, it's helpful to look at the long-held theory that it may replace.
Earth's Water: The Widely-Held Theory
For a long time, most scientists believed the water-from-comets-and asteroids version of water's origin here on Earth. It all starts with the formation of the Sun.
When the Sun formed out of a molecular cloud, it swept up most of the material in the cloud, leaving a little left over for everything else: planets, asteroids, and comets. Once the Sun burst into life with fusion, a powerful solar wind sent a lot of hydrogen from its outer layers out beyond where the inner rocky planets—Mercury, Venus, Earth, and Mars—are today.
This is the realm of the gas giants, and more importantly, comets and asteroids. Comets are icy, rocky bodies, thought to contain significant amounts of the hydrogen blown out there by the early Sun, and asteroids too, although to a lesser extent. They became a significant reservoir for hydrogen.
[caption id="attachment_140480" align="alignnone" width="525"]
An artist's conception of the dust and gas surrounding a newly formed planetary system. Somewhere in there is Earth's water. Credit: NASA[/caption]
When Earth formed, it was a molten ball, its surface kept in that state by repeated collision with asteroids. So far, so good, since the early Solar System was a much more chaotic place than it is now. As asteroids and comets struck this hot Earth, the water and the hydrogen in it were boiled off into space. As the Earth cooled over time, water from comet and asteroid collisions was allowed to condense on Earth, and not be boiled off into space. The water stuck around.
The evidence for this lies in isotope ratios. The ratio of the heavy hydrogen isotope deuterium to normal hydrogen is a chemical signature. Two bodies of water with the same ratio must have the same origin, the thinking goes. And Earth's oceans have the same ratio as water on asteroids.
That's a very simplified version of the widely-held theory of how water got to Earth.
Earth's Water: A Leaky Theory With Holes In It
But scientists are malcontents, always trying to have a better, more thorough understanding of things. They were questioning the "water from comets" theory before this newest study came out.
Back in 2014, some
scientists studied
the issue by looking at meteorites of different ages. (Meteorites are just asteroids that have struck Earth.) First they looked at what are known as 'carbonaceous chondrite meteorites'. They're the oldest ones we know of, and they formed about the same time as the Sun did. They're the primary building blocks of Earth.
Next, they studied meteorites that we think originated from the large asteroid Vesta.
Vesta
formed in the same region as Earth, about 14 million years after the solar system was born. According to this 2014 study, the ancient meteorites resembled the bulk Solar System composition and have a lot of water in them, so they've been widely considered to be the source of Earth's water.
[caption id="attachment_139410" align="alignnone" width="700"]
The asteroid Vesta, courtesy of NASA's Dawn spacecraft. Meteorites ejected from Vesta may have helped form Earth's water. Credit: NASA/JPL-Caltech/UCAL/MPS/DLR/IDA [/caption]
The measurements in this 2014 study showed that these meteorites have the same chemistry as the carbonaceous chondrites and rocks found on Earth. They concluded that carbonaceous chondrites are the most likely common source of water. At the time, Horst Marschall, one of the authors of the study, said, "The study shows that Earth's water most likely accreted at the same time as the rock. The planet formed as a wet planet with water on the surface." The team behind that study acknowledged that some of our water did come from impacts.
Which brings us to this new study, which reinforces the conclusions from the 2014 study.
Earth's Water: All About The Hydrogen
The authors of this new study say that the oceans and their isotope ratios may not tell the whole story. "It's a bit of a blind spot in the community," said Steven Desch, a professor of astrophysics in the School of Earth and Space Exploration at Arizona State University in Tempe, Arizona. "When people measure the [deuterium-to-hydrogen] ratio in ocean water and they see that it is pretty close to what we see in asteroids, it was always easy to believe it all came from asteroids." It's hard to blame them; it's a pretty compelling piece of evidence.
"It's a bit of a blind spot in the community." - Steven Desch, School of Earth and Space Exploration, ASU.
Desch and the other authors of this new study point to research
published in 2015
showing that the Earth's oceans may not be representative of Earth's primordial water. The oceans may have cycled between the surface and a deeper reservoir of water, deep in the Earth. This may have changed the ratio over time, and it may mean that this deeper water represents at least some of Earth's true primordial water. And that water may have come directly from the solar nebula, rather than through comet and asteroid impacts.
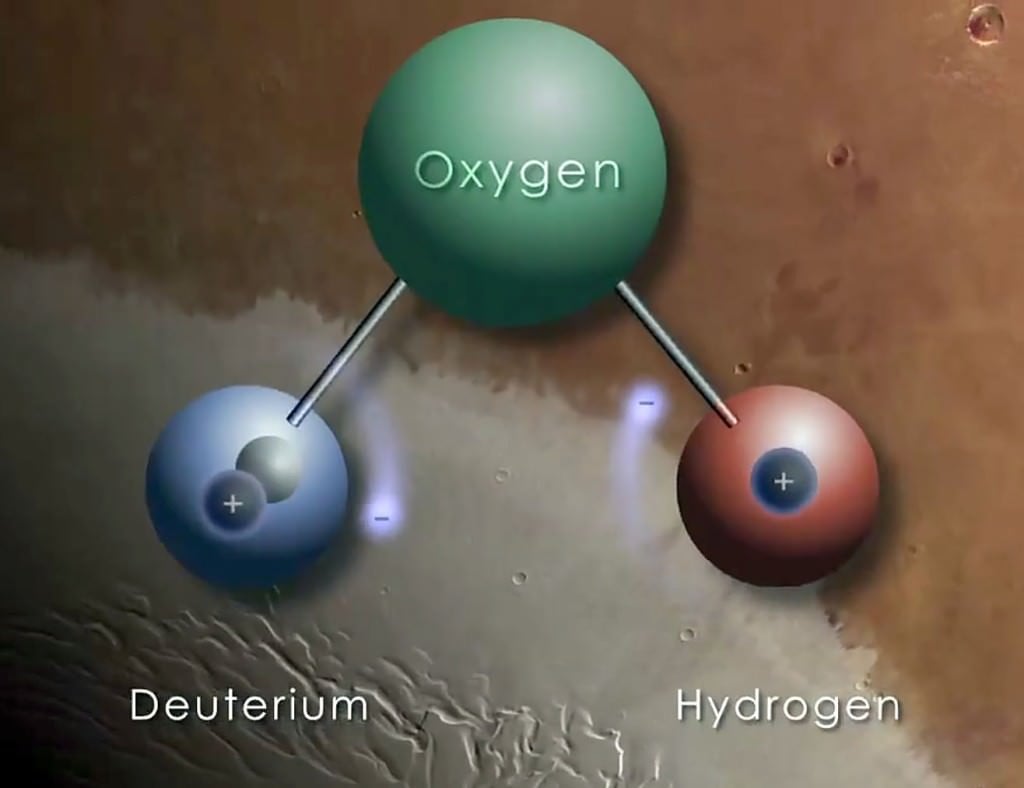
[caption id="attachment_119275" align="alignnone" width="525"]
A hydrogen atom is made up of one proton and one electron, but its heavy form, called deuterium, also contains a neutron. Credit: NASA/GFSC[/caption]
The study develops a new theoretical model of Earth's formation to explain these differences between hydrogen in Earth's oceans and at the core-mantle boundary.
This new model shows large water-logged asteroids formed into planets billions of years ago in the solar nebula swirling around the Sun. These planetary embryos suffered sequential collision and they grew quickly. Eventually, they say, a powerful enough collision melted the surface of the largest embryo into an ocean of magma. This largest embryo became Earth.
This large embryo had enough gravity to hold onto an atmosphere, and it attracted gases, including hydrogen, the most abundant one, from the solar nebula to form one. The hydrogen in the solar nebula contained less deuterium and is lighter than asteroidal hydrogen. It dissolved into the molten iron of the magma ocean on Earth.
The hydrogen was pulled to the center of the Earth by a process called isotopic fractionation. Hydrogen is attracted to iron and was delivered the Earth's core by the iron. Deuterium, the heavy hydrogen isotope, remained in the magma, which cooled to form the Earth's mantle. Continuing impacts brought more water and mass to Earth, until it reached the mass it is today.
The key point in this new model is that hydrogen in the Earth's core is different than hydrogen in the mantle and in the oceans. Core water has much less deuterium. But what does it all mean?
The new model allowed the authors to estimate the amounts of water that came from asteroid impacts as Earth grew and evolved, compared to how much came from the solar nebula when the Earth formed. Their conclusion? "For every 100 molecules of Earth's water, there are one or two coming from solar nebula," said Jun Wu, assistant research professor in the School of Molecular Sciences and School of Earth and Space Exploration at Arizona State University and co-lead author of the study.
Conclusion: It's About More Than Just Earth's Water
This study is a new perspective on planetary formation, development, and on how early life could flourish on a young planet.
"This model suggests that the inevitable formation of water would likely occur on any sufficiently large rocky exoplanets in extrasolar systems. I think this is very exciting." - Jun Wu, School of Molecular Sciences and School of Earth and Space Exploration at ASU, co-lead author.
Previously, we thought that the only planets that could have life on them would have to be in a solar system rich with water-bearing asteroids and comets. But that may not be the case. In other solar systems, not all Earth-like planets have access to asteroids loaded with water. The new study suggests any habitable exoplanets might have gotten water from the solar nebula in their system. Earth hides most of its water in its interior. Earth has roughly two ocean in its mantle, and 4 or 5 in its core. Exoplanets may be similar.
[caption id="attachment_110690" align="alignnone" width="750"]
Artist's impression of a massive asteroid belt in orbit around a star. Earth's water may not have all come from asteroids and comets, so maybe that's true for exoplanets. Credit: NASA-JPL / Caltech / T. Pyle (SSC)[/caption]
"This model suggests that the inevitable formation of water would likely occur on any sufficiently large rocky exoplanets in extrasolar systems," Wu said. "I think this is very exciting."
There's one cautionary point in this new model though, and that involves the hydrogen fractionation. It's not well-understood how the deuterium-to-hydrogen ratio changes when the element dissolves in iron, which is at the center of this new model. It had to be estimated in this new study.
Overall, the new study fits in well with other research into Earth's water. Once more work is done on hydrogen fractionation, the new model can be tested more rigorously.
Sources:
- AGU Press Release: " Scientists theorize new origin story for Earth’s water "
- Research Paper: " Origin of Earth's Water: Chondritic Inheritance Plus Nebular Ingassing and Storage of Hydrogen in the Core "
- Research Paper: " Evidence for primordial water in Earth’s deep mantle "
- Research Paper: " Early accretion of water in the inner solar system from a carbonaceous chondrite–like source "
- Wikipedia: Formation and evolution of the Solar System
- Wikipedia: 4 Vesta
 Universe Today
Universe Today