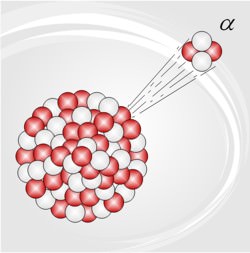
Alpha radiation is another name for the alpha particles emitted in the type of radioactive decay called alpha decay. Alpha particles are helium-4 (4He) nuclei.
Radioactivity was discovered by Becquerel, in 1896 (and one of the units of radioactivity – the becquerel – is named after him); within a few years it was discovered (Rutherford gets most of the credit, though others contributed) that there are actually three kinds of radioactivity, which were given the exciting names alpha (radiation), beta (radiation), and gamma (radiation; there are some other, rare, kinds of radioactive decay, the most important being positron, or positive beta). Rutherford (with some help) worked out that alpha radiation is actually the nuclei of helium … by allowing alpha radiation to go through the thin walls of an evacuated glass tube, and later analyzing the gas in the tube spectroscopically).
Some fun facts about alpha radiation:
* alpha radiation is the least penetrating (of alpha, beta, and gamma); typically it goes no more than a few cm in air
* like all kinds of radioactive decay, alpha decay occurs because the final state of the nucleus (the one decaying) has a lower energy than the initial one (the difference is the energy of the emitted alpha particle, both its binding energy and its kinetic energy)
* alpha decay involves both strong and electromagnetic interactions (or forces), unlike beta and gamma decay
* the key to the specifics of alpha decay is the quantum effect called tunneling; Gamow worked this out, in 1928
* only heavier nuclides can undergo alpha decay; the lightest are light isotopes of tellurium
* alpha radiation played a star role in the development of our understanding of the nature of atoms … Rutherford, in 1909, aimed a beam of alpha radiation at a piece of thin gold foil, and counted the number of particles which were deflected at each angle; from this he deduced that the atom has a very small nucleus (with all the positive charge, and nearly all its mass).
For more background on alpha radiation, check out the Jefferson Lab’s What are alpha rays? How are they produced?.
There are many ways alpha radiation can turn up in Universe Today articles; for example, in NASA May Have to Revamp Science Plans Without RTGs, alpha radiation is essential to RTGs; and in Opportunity Rover Sidelined by Charged Particle Hit, alpha radiation is what’s used to help determine the elemental composition of samples.
Nucleosynthsis: Elements from Stars and Cosmic Rays are two Astronomy Cast episodes which also cover alpha radiation.
Source: Wikipedia