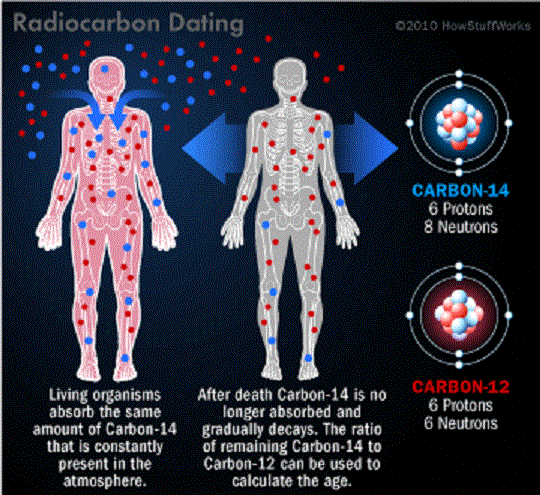
Here on Earth, Carbon is found in the atmosphere, the soil, the oceans, and in every living creature. Carbon 12 – aka. C-12, so-named because it has an atomic weight of 12 – is the most common isotope, but it is by no means the only one. Carbon 14 is another, an isotope of carbon that is produced when Nitrogen (N-14) is bombarded by cosmic radiation.
This process causes a proton to be displaced by a neutron, effectively turning atoms of Nitrogen it into an isotope of carbon – known as”radiocarbon”. It is naturally radioactive and unstable, and will therefore spontaneously decay back into N-14 over a period of time. This property makes it especially useful in a process known as “radiocarbon dating”, or carbon dating for short.
Origin of Radiocarbon:
Radiocarbon enters the biosphere through natural processes like eating and breathing. Plants and animals absorb both C-12 and C-14 in the course of their natural lifetimes simply by carrying out these basic functions. When they die, they cease to consume them, and the isotope of C-14 begins to revert back to its Nitrogen state at an exponential rate due to its radioactive decay.
Comparing the remaining C-14 of a sample to that expected from atmospheric C-14 allows the age of the sample to be estimated. In addition, scientists know that the half-life of radiocarbon is 5,730 years. This means that it takes a sample of radiocarbon 5,730 years for half of it to decay back into nitrogen.
After about 10 half-lives, the amount of radiocarbon left becomes too minuscule to measure and so this technique isn’t particularly reliable for dating specimens which died more than 60,000 years ago – i.e. during the late Middle Paleolithic (aka. Old Stone Age) period.
History of Development:
Experiments that would eventually lead to carbon dating began in the 1939s, thanks to the efforts of the Radiation Laboratory at the University of California, Berkeley. At the time, researchers were attempting to determine if any of the elements common to organic matter had isotopes with half-lives long enough to be of value in biomedical research.
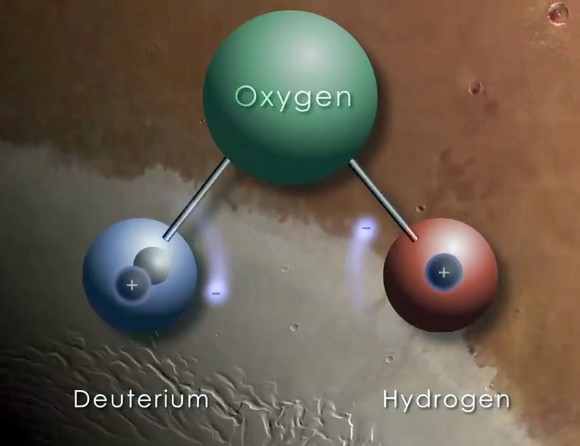
By 1940, the half-life of Carbon 14 was determined, as was the mechanism through which it was created (slow neutrons interacting with Nitrogen in the atmosphere). This contradicted previous work, which held that it was the product of deuterium (H², or heavy hydrogen) and Carbon 13.
During World War II, Willard Libby – a chemist and graduate of Berkeley – read a paper by W. E. Danforth and S. A. Korff (published in 1939) which predicted that C 14 would be created in the atmosphere due to interactions between nitrogen and cosmic rays. From this, Libby came up with the idea of measuring the decay of C 14 as a method of dating organic material.
In 1945, Libby moved to the University of Chicago, where he began the work that would lead to the development of radiocarbon dating. In 1946, he published a paper in which he speculated that C 14 might exist within organic material alongside other carbon isotopes.
After conducting experiments, which measured C-14 in methane derived from sewage samples, Libby and his colleagues were able to demonstrate that organic matter contained radioactive C-14. This was followed by experiments involving wood samples for the tombs of two Egyptian kings, for which the age was known.
Their results proved accurate, with allowances for a small margin of error, and were published in 1949 in the journal Science. In 1960, Libby received the Nobel Prize in Chemistry for this work. Since that time, carbon dating has been used in multiple fields of science, and allowed for key transitions in prehistory to be dated.
Limits of Carbon Dating:
Carbon dating remains limited for a number of reasons. First, there is the assumption that the ratio of C-12 to C-14 in the atmosphere has remained constant, when in fact, the ratio can be affected by a number of factors. For instance, C-14 production rates in the atmosphere, which in turn are affected by the amount of cosmic rays penetrating the Earth’s atmosphere.
This is itself affected by things like the Earth’s magnetic field, which deflects cosmic rays. Furthermore, precise measurements taken over the last 140 years have shown a steady decay in the strength of the Earth’s magnetic field. This means there’s been a steady increase in radiocarbon production (which would increase the ratio).
Another limitation is that this technique can only be applied to organic material such as bone, flesh, or wood, and can’t be used to date rocks directly. On top of that, the addition of Carbon 12 will throw off the ration, thus leading to inaccurate assessments of a sample’s age.
This is where anthropogenic factors come into play. Since fossil fuels have no Carbon 14 content, the burning of gasoline, oil, and other hydrocarbons – and in greater and greater quantity over the course of the past century and a half – has diluted the C-14 content of the atmosphere.
On the other hand, atmospheric testing of nuclear weapons during the 1950s and 1960s is likely to have increased the Carbon 14 content of the atmosphere. In fact, research has been conducted which suggests that nuclear tests may have doubled the concentration of C-14 in this time, compared to natural production by cosmic rays.
Nevertheless, it remains the most accurate means of dating the scientific community has discovered so far. Until such time that another method becomes available – and one that produces smaller margins of error – it will remain the method of choice for archeology, paleontology, and other branches of scientific research.
We have written many articles about Carbon Dating for Universe Today. Here’s How Do We Know How Old Everything Is?, How Old is the Universe?, How Old is the Solar System?, How Long has Humans been on Earth?
If you’d like more info on Carbon Dating, check out NASA’s Virtual Dating: Isochron and Radiocarbon – Geology Labs On-line, and here’s a link to USGS Radiometric Dating Page.
We’ve also recorded an entire episode of Astronomy Cast all about How Carbon Dating Works. Here’s Episode 122: How Old is the Universe? and Episode 164: Inside the Atom.
Sources:

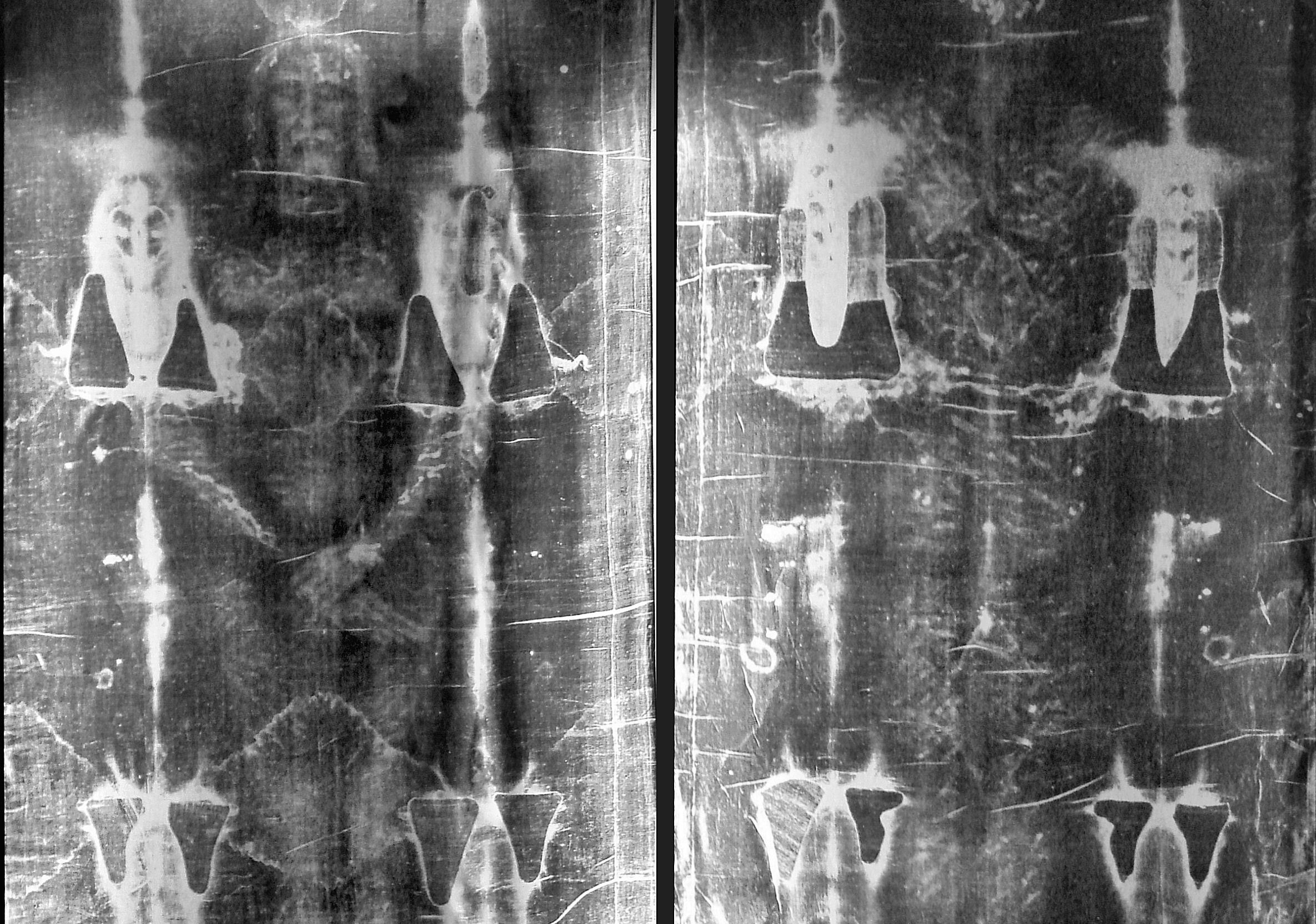
You showed a picture of the turin shroud which was carbon dated to the 13th century. There was much controversery over this as the sample they used was from an area of cloth where the priests would have held it when it was publicly displayed, raising the issue of contamination.