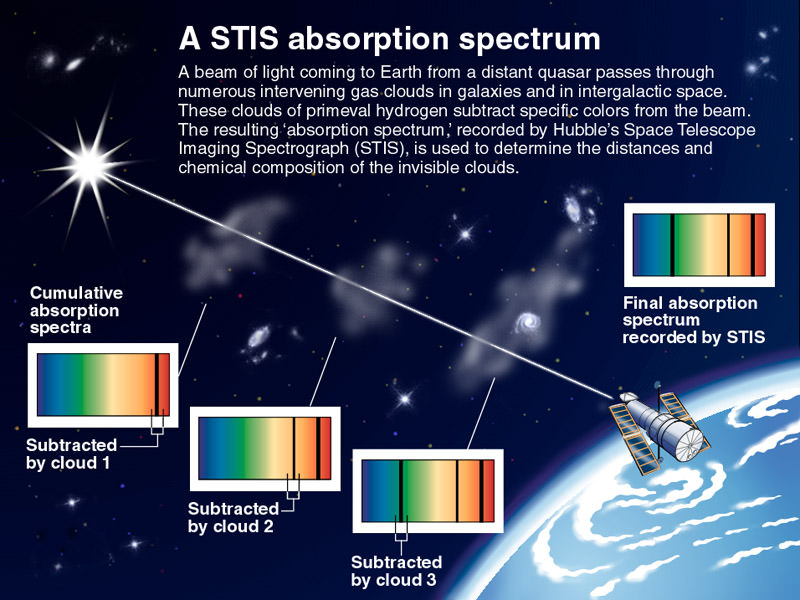
[/caption]
In terms of physics, absorption is defined as the way that energy from photons is taken up by matter, and transformed into other forms of energy, like heat. All of the light in the electromagnetic spectrum is made up of photons at different energy levels. Radio waves are photons with lower amounts of energy, and gamma rays are photons with very high levels of energy. When a photon strikes matter, it can either be reflected or absorbed by the material. And if it is absorbed, the energy of the photon is transformed into heat.
The absorbance of an object is a measure of what percentage of the electromagnetic radiation it’s likely to absorb. Transparent or reflective objects absorb much less than opaque, black objects.
This concept is very important to astronomers, who are able to measure which wavelengths of light are being absorbed by an object or cloud of gas, to get an idea of what it’s made of. When you put the light from a star through a prism, you get a spectrum of the light coming from that star. But in some spectra, there are blank lines, gaps where no photons of a specific wavelength are being emitted. This means that some intervening object is absorbing all of the photons of this wavelength.
For example, imagine looking at how the light from a star passes through a planet’s atmosphere which is rich in sodium. This sodium will absorb photons at a specific wavelength, creating gaps in the spectrum from the light of the star. By comparing these gaps to the absorption line pattern of known gasses, astronomers can work out what’s in the planet’s atmosphere. This general method is used in many ways by astronomers to learn what distant objects are made out of.
The opposite of absorption is emission. This is where different elements will release photons when they’re heated. Different elements will release photons at different levels of energy, and their colors on the electromagnetic spectrum help astronomers discover what elements the object is made out of. When iron is heated, it releases photons in a very specific pattern, different from the pattern released by oxygen.
Both the absorption and emission serve as a fingerprint to help astronomers understand what the Universe is made out of.
We have written many articles about Absorption Spectroscopy for Universe Today. Here’s an article about amateur spectroscopy, and here’s an article about the light spectrum.
If you’d like more info on Absorption Spectroscopy, check out the Principles of Spectroscopy, and the Infrared Spectroscopy Page.
We’ve also recorded an episode of Astronomy Cast all about the Hubble Space Telescope. Listen here, Episode 88: The Hubble Space Telescope.
Source:
Wikipedia