[/caption]
The production of elements in supernova explosions is something we take for granted these days. But exactly where and when this nucleosynthesis takes place is still unclear – and attempts to computer model core collapse scenarios still pushes current computing power to its limits.
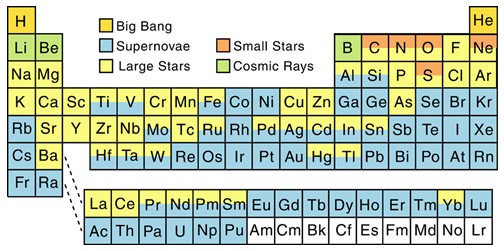
Stellar fusion in main sequence stars can build some elements up to, and including, iron. Further production of heavier elements can also take place by certain seed elements capturing neutrons to form isotopes. Those captured neutrons may then undergo beta decay leaving behind one or more protons which essentially means you have a new element with a higher atomic number (where atomic number is the number of protons in a nucleus).
This ‘slow’ process or s-process of building heavier elements from, say, iron (26 protons) takes place most commonly in red giants (making elements like copper with 29 protons and even thallium with 81 protons).
But there’s also the rapid or r-process, which takes place in a matter of seconds in core collapse supernovae (being supernova types 1b, 1c and 2). Rather than the steady, step-wise building over thousands of years seen in the s-process – seed elements in a supernova explosion have multiple neutrons jammed in to them, while at the same time being exposed to disintegrating gamma rays. This combination of forces can build a wide range of light and heavy elements, notably very heavy elements from lead (82 protons) up to plutonium (94 protons), which cannot be produced by the s-process.
Prior to a supernova explosion, the fusion reactions in a massive star progressively run through first hydrogen, then helium, carbon, neon, oxygen and finally silicon – from which point an iron core develops which can’t undergo further fusion. As soon as that iron core grows to 1.4 solar masses (the Chandrasekhar limit) it collapses inwards at nearly a quarter of the speed of light as the iron nuclei themselves collapse.
The rest of the star collapses inwards to fill the space created but the inner core ‘bounces’ back outwards as the heat produced by the initial collapse makes it ‘boil’. This creates a shockwave – a bit like a thunderclap multiplied by many orders of magnitude, which is the beginning of the supernova explosion. The shock wave blows out the surrounding layers of the star – although as soon as this material expands outwards it also begins cooling. So, it’s unclear if r-process nucleosynthesis happens at this point.
But the collapsed iron core isn’t finished yet. The energy generated as the core compressed inwards disintegrates many iron nuclei into helium nuclei and neutrons. Furthermore, electrons begin to combine with protons to form neutrons so that the star’s core, after that initial bounce, settles into a new ground state of compressed neutrons – essentially a proto-neutron star. It is able to ‘settle’ due to the release of a huge burst of neutrinos which carries heat away from the core.
It’s this neutrino wind burst that drives the rest of the explosion. It catches up with, and slams into, the already blown-out ejecta of the progenitor star’s outer layers, reheating this material and adding momentum to it. Researchers (below) have proposed that it is this neutrino wind impact event (the ‘reverse shock’) that is the location of the r-process.
It’s thought that the r-process is probably over within a couple of seconds, but it could still take an hour or more before the supersonic explosion front bursts through the surface of the star, delivering some fresh contributions to the periodic table.
Further reading: Arcones A. and Janka H. Nucleosynthesis-relevant conditions in neutrino-driven supernova outflows. II. The reverse shock in two-dimensional simulations.
And, for historical context, the seminal paper on the subject (also known as the B2FH paper) E. M. Burbidge, G. R. Burbidge, W. A. Fowler, and F. Hoyle. (1957). Synthesis of the Elements in Stars. Rev Mod Phy 29 (4): 547. (Before this nearly everyone thought all the elements formed in the Big Bang – well, everyone except Fred Hoyle anyway).

I would love to hear Hon. Saculous B Crumb interpretation of a simple detonation of a type Ia supernova and what elements are created-and spread throughout the Universe. His interpretation is very clear to me. Most other interpretations I’ve read are confusing to me. I am always very interested in this very unusual and very rare event.
Assuming the typical Type Ia supernova is Mv= -19.3, settting it to the distance of Alpha Centauri would be ~ -23.5 and a distance of 3260LY -9.3, I hope to see a type Ia supernova within 5000LY in my lifetime.
I find it odd that Li, Be and B are formed from cosmic rays. I should think that very little of these elements would exist. I think some Li was generated in the big bang. Yet these elements are relatively common.
LC
@LBC
The table presented refers to the main source of the elements. Lithium and beryllium were formed in the BB, but most of that present in the universe today 😉 was formed later through cosmic ray spallation.
http://en.wikipedia.org/wiki/Nucleosynthesis#Cosmic_ray_spallation
So why didn’t Big Bang create heavy elements? Was it all quarks or what?
@headaroundu
The BB never had the right combination of heat and density. It was cool enough for protons to form after the first three minutes – but there was only another 17 minutes for fusion reactions to take place before the universe had expanded and cooled too much. From there, its baryonic matter content was about 75% hydrogen, 25% helium and traces of lithium and beryllium – everything else subsequently came from stars (apart from all the dark stuff anyway).
@HeadAroundU : This is largely because the time period where temperatures in the big bang were in the right range to cook up heavy elements lasted a few seconds. It was also not a period of implosive pressure as in the interior of a collapsing star, but a time where material was expanding outward. So the period of equilibrium for p + n –> D was brief and far to short to cook up heavier elements. It is possible that a few carbon nuclei, maybe an iron or lead nucleon here and there were cooked up out of 10^{30} or 10^{40} nucleons (protons, deuterium and helium). However, largely deuterium and helium were all that emerged from big bang fusion.
LC
Steve is right, and for some reason I said seconds, when I should have said minutes.
LC
the big-bang could resemble a self-similar quantum field effect supernova event horizon collision explosion that formed hierarchial fractal scale patterns from atoms, stars, galaxies etc. everywhere still today. each scale has intrinsically a brief period of time relativity that heavier elements can form until expanding cooling beyond further allowable conditions. The fact that the LHC is colliding particles like in a stars reactor, to study big-bang conditions, and claiming to search for the higgs boson and god particles, indicates that the universe is a quantum effect big-bang.
Jackson says both the early mantle and the Baffin Island lava rocks, have tell-tale neodymium isotopes that are unlike chondrites. Also a very old lead isotope signature and helium 3 levels were found. So the moon did not come from the earth by a collision. The moon has one ten thousandeth 1/10,000 the total Hydrogen content of the earth by recent measurements showing its devoid of water unlike the earth in early solar system formation. Up to 10 percent holy grail lava basalts might still exist on earth, found in non-geothermal void spaces between tectonic plates, and not uplifted from hot spots that melt rocks. Preservation beneath permanent canadian glacier ice caps and being deeply beneath the surface kept them away from erosion and metamorphism. Please inform of the toe (theory of everything) model that relates this discovery with big-bang nucleosynthesis theory. Its interesting that red giants before supernova have a nickel iron core much like earth that is turned inside out as exploding elements expands and cools, iron from supernovas is found on earths ocean floor.
During the big bang the first particles moved at relativistic speeds. Did this have any effect on the forming event of the Big bang? For example weighing more heavy and time dilation?
Thanks for answers.
So, only 17 minutes for a slow process.
LC, you mean iron or lead nukleus, right? Nucleons are only protons and neutrons.
Every picture tells a story…. http://helios.gsfc.nasa.gov/onion.gif
The caption in the image above that says the most, is: “Nuclear burning occurs at the boundaries between zones.” aka… pressure/density stratification in the magnetically and gravitationally constrained expanding fusion plasma(s).
This says little about ‘cold flow’ plasmas or those plasmas which are magnetically constrained but not restricted to a gravitationally confined condensate which may indicate weak force interactions within the nuclear force via electromagnetically enhanced confinement. ‘Cold flow’ in this case being temperatures below the minimum temperature required for the fusion of hydrogen at 5 million degrees.
HeadAroundU
Yes I meant nuclei, I was a bit tired when I wrote that and kept making slips.
LC
Steve
Thank you for this excellent UT story on stellar evolution and nucleosynthesis by-products. It is a good summary of the current level of knowledge on this interesting subject, which frankly I have trouble faulting. (If I were to be honestly critical, the presentation might be just a tad too complex for the average reader here – perhaps trivial in some respects.
Of course, the main products being made are mostly formed in ordinary stars and not so much in supernovae – though most elements heavier than 56 Iron are certainly made in these catastrophic events.
One particular issue that I find more interesting is the distribution and numbers of each of the element’s isotopes. Knowledge of these products tells much about how these elements were formed – assumed from environments of poor or rich in neutrons. (I.e. Like an example story I recently read on “Neutron-rich and double magic: nucleus is a first” and reviewed in Physical Review Letters Phys. Rev. Lett. 102, 152501 (2009) ) I also think that information of the broken fragmented by-products of elements created by radioactive decay also tell much about the nature of the processes.I.e. Nickel, Cobalt and Iron production in supernovee.
If we were to say something of the future in discovering the nature of the alchemy. The paper of Arcones and Janka is only the tip of the iceberg when it comes to the future of this field of astrophysics.
NOTE: There is an interesting story available on AstronomyOnline.org which might interest some UT reader. The article is Nodelling Core Collapse Supernova by Alex Nervosa.
This gives an excellent picture of the theoretical and observational evidence of nucleosynthesis models. Highlighted text within the section “Nucleosynthetic Chemical Yields & Mass Ejection” gives a good deeper explanation of the UT story here.
LBC said;
“I find it odd that Li, Be and B are formed from cosmic rays. I should think that very little of these elements would exist. I think some Li was generated in the big bang. Yet these elements are relatively common.”
I disagree. In relative abundances, Lithium (actually Li-7) is still fairly rare among the distribution of elements. This primordial lithium after a star is formed is rapidly destroyed and therefore is near impossible to exist from shining stars to supernovae. However, lithium stars (or more properly; lithium-line stars) do exist. I.e The 8.9 magnitude star HIP 99423 / HD 345957 in Vulpecula (Weird Spectral Type of G0Vw )
A technical arVix paper by C.Charbonnel, F.Primas is;
The Lithium Content of the Galactic Halo Stars (A&A., 442, 961 (2005) [It should answer all your question here, and gives useful discussions of what is available in the literature.]
Simply. Li-6, as Steve has already said, is caused by spallation with cosmic rays. Li-7 can also be made on the boundary layer of the stellar core where Be-7 is converted by electron capture to 7-Li – then is rapidly transferred to the stellar surface via normal convection. (This is how we see these lithium stars!)
Cheers
Wow! Let me say that again: wow! That is quite possible the most poignant and beautiful picture I’ve seen! (I.e., in the last months, seeing as how our minds work. :-D)
It was also quite easy to understand the illustrated bounce back symmetry inside the earlier ejecta from the text and the reference. (Its fig 1, especially the more symmetric right column.) Thank you, Steve!
People here have hashed out nucleosynthesis to everyone’s satisfaction I think. A datum I seem to remember is that big bang (BB) nucleosynthesis lithium used to be considered deficit in old (pop I ?) stars by a factor 2 or so, but IIRC newer models shows how it has preferentially settled “out of sight” spectroscopically and tested yet again standard cosmology predictions.
So what can I do but continue to wax poetically. As we all know, our solar system shows signs to have been birthed by a cloud that was compressed, or at least seeded, by a supernova, leaving its telltale marks of relatively high metallicity.
This has, AFAIU, been a little question mark as far as planetary formation goes, too little metallicity and presumably fewer planets, too much metallicity and other barriers for planet formation. It is a fun exercise to peruse an exoplanet database, ask for planets by star metallicity, and see that the Sun even so places among the maximum in the distribution of “planet producing stars”.
This is a marginal toe in to last weeks falling-on-my-but-in-perplexity press release. Researchers have found Earth material that are as old as Earth itself!
Alas not crust, but mantle material, which reservoir dates back to a few tens of millions of year after Earth formation. It has been punctuated and dragged to the surface on Baffin Island by those mysterious deep hotspots. Here it is Iceland’s hotspot that used to reside there 60 Ma.
The reservoir mantle material is dated 4.55 – 4.45 Ga, Earth at 4.55 Ga. But the accompanying model shows that its composition is explained by a rapid quench of a Earth crust over 30 My, coinciding with Earth differentiation and core formation.
This iron rich material was then too heavy, broke up and sunk, leaving the mantle composition to be the Baffin island one.
There are many consequences drawn from this single and, to me, unexpected discovery. Among others, the models of Earth/Moon as primordially chondrite material is shored up in the original paper, as well as models for early core formation even in small bodies. I believe, but its iffy to say for a layman, that the Earth/Moon impactor vapor homogenization model is also shored up, explaining the exact O and W ratio match between Earth and Moon.
But, bother! If we can find mantle material from up to the initial Earth formation, why is the crust history “broken” the earliest 0.7 Gy? (Oldest found crust is 3.8 Ga. Some zircon crystals are 4.4 Ga though.)
Now Carlson holds out the alluring possibility that the first crust is still to find in reservoirs, but in molten form explaining seismological observations. Maybe so, but that is a long stretch. And even molten, I’ll take that morsel, but we can’t get to it! Where is a hotspot when you need it?
Yes, no, yes, no. Deficit, pop 2, a factor ~ 3, and Salacious paper (thanks!) shows that the debate is ongoing. I must have read one specific paper at some time.
@JIMHENSON,
It appears that your philosophy is: Everything about science that I needed to know I learned from watching cheap sci-fi shows.
(Excuse me, everyone else, but I’ve had a bit too much beer and it needed saying!)
Oh, how deeply I failed in my attempt to wax poetically!
First, I should have pondered our presumably fiery birth by supernova. Seeing that our galaxy start birth rate is ~ 1 y^-1, but supernovas rate is ~ 0.01 y^-1, a _very_ loose statistic says that our Sun is not so unique anyway, around the percent range likely.
Second, about Earth early crust. If the first KREEP like crust (after the Moon K, Rare Earth Elements, P rich lava sea condensates) was made and gone in ~ 30 My, @ 4.52 Ga, the story gets interesting.
The mantle find is, as I noted, presumably indicative of a vaporizing impact @ ~ 4.48 Ga making the Moon. Latest figures as I get them, adding another 40 My of story, and now fair odds of erasing a second solidified crust. Maybe we will find impact broken pieces on the Moon some day, settling in the gravity well after the main mass did.
Then we have a new crust, the 2nd or 3d one, and liquid water @ ~ 4.404 +/- 0.008 Ga as assured by zircons. That is another 80 My, now comfortable given the new time constants.
Seems Earth is an old crusty planet. Was the 3d time the charm?
Salacious: I have not devoted much study to nucleosynthesis, so the idea that Li and Be emerged from cosmic ray fission has come as news to me. According to
http://en.wikipedia.org/wiki/Nucleosynthesis#Cosmic_ray_spallation
Li is rarer than I thought, but it is not much rarer than copper. Anyway, this article did illuminate something I was pretty unaware of, which is that cosmic rays play a role in nucleosynthesis.
LC
I am very interested in various SN and thank those who posted about various SN.
My career did not cover Pro Astronomy, Astrophysics, etc. I’m just an amateur Astronomer who had a career in Computer Sciences. I am aware of very massive stars creating gamma-ray jets beaming long distance when the massive star detonates, a beamed jet aimed staight at Earth from 10000LY will be harmful to our lifeforms.. A question concerns SN2007bi, the 1st and only pair-instability ultra-supernova explosion known. It appears this ultra massive star creates antimatter pairs of electron positrons causing the entire mass of perhaps 300X solar mass to continualy destroy itself for a prolonged detonation period until the ultra massive star is completely gone.It appeared no jets were created. My question is, should a super-massive star ending its’ life in this manner be a danger to Earth should it occur 10000LY away??
I’m aware these super-stars are very rare and mostly gone from our Universe, with the exception perhaps R136a may harbor a super-star or few in the 150-300solar mass. I’m aware there is much we don’t know about our hugh Milky Way galaxy.and many unusual objects are still to be discovered.
@ Olaf:
I suspect this is more LBC expertise, but let me bumble around this meanwhile.
Your question touches on high curvature general relativity, because if you follow the expansion process long enough backwards into inflation, our observable universe comes out of a very small volume. (Even down to Planck size in high field chaotic inflation, AFAIU.)
This is problematic, and I don’t know how much is known. But in general particles are objects of “low energy” fields, so uncoupled from the previous high curvature (high gravity energy) process. They should freeze out well after “the forming event”, and indeed that is how it is popularly described. (Say, Wikipedia or books like “A Brief History of Time” by Hawking.)
@ ILoveTheStar:
I believe you refer to gamma-ray bursts, and I don’t know much about them, only that they are suspected to be tied to supernovas.
I do know something about the biosphere, at an interested layman level. When you say ” harmful to our lifeforms” it can be qualified. A close GRB will punch out the ozone layer and initiate chemical reactions resulting in compounds like nitrous oxides which are harmful. But this will at most make a mass extinction event.
The Earth has weathered many mass extinction events, life is really robust. Moreover, diversity tends to increase after them. Modern research believes diversity recovery times can even be on time scales of < ~ 1 My, which is a few times the typical mammal speciation time (think polar bears, ~ 0.1 My) and about the typical mammal species life time.
So a close GRB directed towards us (rather narrow cone) may be a bummer for city life and the next vacation, but it is hardly threatening for our biosphere. Closer than 10-100 ly it could perhaps knock out all life, but then again the Sun going red giant will do that in ~ 0.2 – 1 Gy anyway while we have wintered 4 Gy worth of biosphere life without any GRB final kill.
In sum, it's a danger roughly the equivalent of worrying that you personally will be hit by a meteor. I wouldn't obsess about it. Chances are you will die in a bed. Entertaining two blond twins, hopefully.
“If the first KREEP like crust” – If the first KREEP like crust/mantle system. (It’s the resulting mantle that is KREEP like, obviously.)
@ Jim Henson:
Thanks for your response. The rest is incomprehensible [sorry IVAN3MAN_AT_LARGE, but scifi techno-babble is at least that, on the surface], but this:
Yes, that was the point, they can now predict the discrepancy. This, as well as the KREEPiness, helps, not thwarts, the vapor homogenization model.
Actually, let me qualify that, despite having gushed about these amazing results already.
On the astrobiology course I’m taking, where Earth is used as case study (what else? :-o), I was skeptical when this came up. I couldn’t see how that worked on a microscale. In fact, I’m not sure my descriptive term is the correct one; the chapter is buried in my study stack.
However, at the time I was not aware that the Moon was so differentiated. It was first after I got material on how that predicts the Moons remnant and localized magnetism. (As result of, the putative first, mantle turnover rise.) I believe it has been discussed here. Of course that resets the mineral clock.
Also, I’m not doing research anymore, but I’ve become a predictive hound. First and foremost because it is a first order theory of science, despite the peesky everyday fuzziness of too much or too little data. And precisely because it works well for distant-from-details armchair analysis. And hey, I wouldn’t knock a method that is used to sort out the parameters of the standard model or the topology of the standard phylogeny (both with the help of parsimony).
Finally, a professional astronomer and the astrophysicist lecturer were both excited by it at the time. Granted it wasn’t their area of expertise, but who am I to knock the judgment of professionals? So tentatively I’ve come over on their side, without really knowing too much about the matter,
Further, all this is incidental to the awesomeness of the find. Come on, those guys _predicted. looked for, and found, pieces of Earth that hails back to right after it shook of the interplanetary dust to catch a glimmer of sunlight!
And this after all previous material is nearly 1 Gy older. I’m really curious about Earth formation and how life got started here. I hope that one day get to look at a impactor ejecta rock record that surely can be found on the Moon surface or perhaps even the more stable L4 and L5 gravity pockets.
The Mars rock ALH84001 relatively pristine condition shows how much can be learned from the time arose from the ~ 5 % impact ejecta that shoots out of the gravity well from a hypervelocity impactor like an asteroid; and the Last Heavy Bombardment should have ensured that.
Meanwhile I have to be patient with haphazard finds like this. I’ll easily put it over the same time reported putative find of tool use pushing it back 0.8 My to 3.4 Ma, and perhaps placing Australopithecus as tool user for sure. I mean, come on, even the anthropologists that are skeptical tells us that such a gap isn’t much in the sparseness of the fossil record.
But this! Who ordered this!?
With respect to the question Olaf raised, during the early universe mss-energy existed in an extremely hot plasma. The plasma was at early enough time much hotter than the interior of stars, and hotter than the interiors of neutron or quark stars. Elementary particles, principally quarks and leptons scattered off of each other with large transverse momenta or energy, which enters into a Boltzmann distribution of energy states for an extremely hot gas. Eve further back in time the vacuum structure of the universe was at a higher energy during inflation.
The discussion on neodymium and the distribution of elements enters into territory I find odd frankly. The question of where Earth’s water and oxygen comes from is relevant, for the distribution oxygen isotopes in comets is different from what we have here. I think this boils down to some complexity on how the solar system and the material disk around the formed. It is likely the heavier elements in the solar system did not come from one supernova event. SN remnants tend to push into pre-existing material, which came from other nova or supernova processes. This may have given rise to distributions of elements and isotopes, where each SN event may produce different distributions of elements.
LC
Some heavier, trans-uranic elements certainly were produced naturally. For example decay products of 244Pu (half-life ~80 Myr) occur in many meteorites.
Also, the oxygen isotopic composition varies among many planetary objects, including different types of metorites.
The article at the site below contains a plot of relative abundance of the elements in the solar system. The trend is decreasing abundance with increasing mass and Z (atomic number). Note the odd-even effect whereby elements with even Z are more abundant than those with odd Z. Also note the huge dip in abundance for Li, Be, and B. How these elements were formed was long a big puzzle, as no stellar source seem reasonable. This mass “gap” also produces something of a barrier to element formation from H and He, as element synthesis cannot pass through it by simply adding a proton or neutron. Rather, the temperature must rise such that He nuclei can be added.
http://en.wikipedia.org/wiki/Abundance_of_the_chemical_elements
Could nucleosynthesis someday transmute or fuse base metals into gold by cold fusion of deuterium? Using ocean sea water and copper wires or graphene to superconduct electricity perhaps at ultra low temperatures and magnetically constrained? Some scientists in the past were visited by authorities which lead to abandoning the idea. Do we know more today enough to make cheap Gold, but the world gov’s that control the news prevent this discovery? Some universities are teaching cold fusion, but they might be the very ones to prevent the world from putting it to use for society betterment by keeping it in the hands of wealthy.
Something overlooked? Given that some elements may become superconducting at low temperatures and high pressures within say, planetary sized masses, it may eventually be found that cold flow nucleosynthesis occurs within magnetically constrained and electron polarizing shock fronts? Just saying… there is more that we don’t know than what we think we know?
I’m really liking the Russian experiments aboard the ISS which found crystalline shaped plasmas created in their zero gee vacuum chamber. More por favor!
http://www.mpe.mpg.de/pke/index_e.html
Government conspiracies preventing the development of cold fusion? Yeah, right(!).
If that is the case, then what is stopping the Chinese from developing it, hmmm?
🙄
Furthermore, if you could produce gold cheaply, it would result in its market value dropping like a lead balloon and render its production economically unviable — which would defeat the purpose of trying to produce gold in the first place!
@JimHenson – When I mentioned “cold flow nucleosynthesis” I am referring to temperatures below 5 million degrees which might be induced by the electrostatic acceleration of ions passing through a series of alternately polarized magnetic shock fronts.
The ‘cold fusion’ I think you are referring to was touted by Pons and Flieshman back in the 80’s as a ‘new’ kind of fusion reaction. Actually, what they replicated was done in Germany back in the late 1800’s… as a type of cigarette lighter.
Granted, voluminous output of hydrogen occurs when electrodes of palladium and gold are electrified in D20 (Heavy water)… but still, no neutrons were ever detected from that reaction. Neutrons being the proof of fusion. Never the less, there were some very interesting experiments performed by those wishing to use that reaction for alternative energy generation. Too bad the cost of palladium and gold is so high… making the idea not economically feasible.
There is a lot of quasi-physics that seems to have crept in here. Cold fusion is wrong. The idea one can fuse deuterium in palladium rods is false. The thought occurred to me, though I could not make it work, at a Bose Einstein condensate of deuterium might put all the deuterium ions in the same state and enhance the a quantum transition into helium. However, that is a bit of a wild conjecture on my part. Forget ideas of cold fusion. Nobody seriously considers the idea, and most of these schemes thrown around these days amount to “crank physics.”
If we had access to vast supplies of energy, say from quantum black holes that convert mass 100% into energy we could make any element we wanted. With enough energy you can in principle cook up any element you wanted. So a pile of useless trash could be make into gold bullion. However, if we started filling up the world with gold I would imagine the price of the stuff would plummet.
LC
@LBC – you are correct…. BUT there are continuing ‘cold fusion’ experiments ongoing. Google ‘cold fusion’ and you’ll see what I mean. Wikipedia has a fair description: http://en.wikipedia.org/wiki/Cold_fusion
THIS page has some interesting links… even if you don’t like the name… aka ‘Cold Fusion Times’ http://world.std.com/~mica/cft.html
The chinese are trying to avoid the world trade currency markets, and be isolationistic, hoping to take over, or dominately control the world at the expense of its citizens. money transfers out of every country into big banks all over the world part of the global exchange. s society should benefit greatly by making gold cheap, retail all prices go much lower. I’ve read articles about nanosized black holes in graphene structures particularly carbon nanotubes, that when near absolute zero resemble a black hole…but they’re not actual black holes but have orbiting ions around a charged nanotube moving at relative speeds of light by magnetic fields in experiment. Nothing to make fools gold or pyrite out of.
the vapor homogenization model accounts for the moon being devoid of water relative to the earth, from a collision that formed the moon about 4 billion years ago, when the baffin lava rocks were molten. The water on the moon was vaporized, and it lacks heavier elements found deeper in the earth. I don’t understand KREEP or how the neodymium discrepancy can be determined? Chondrites have recently been proven younger then the baffin lava flows, which means the earth and moon formed before comets and asteroids did in the solar system..