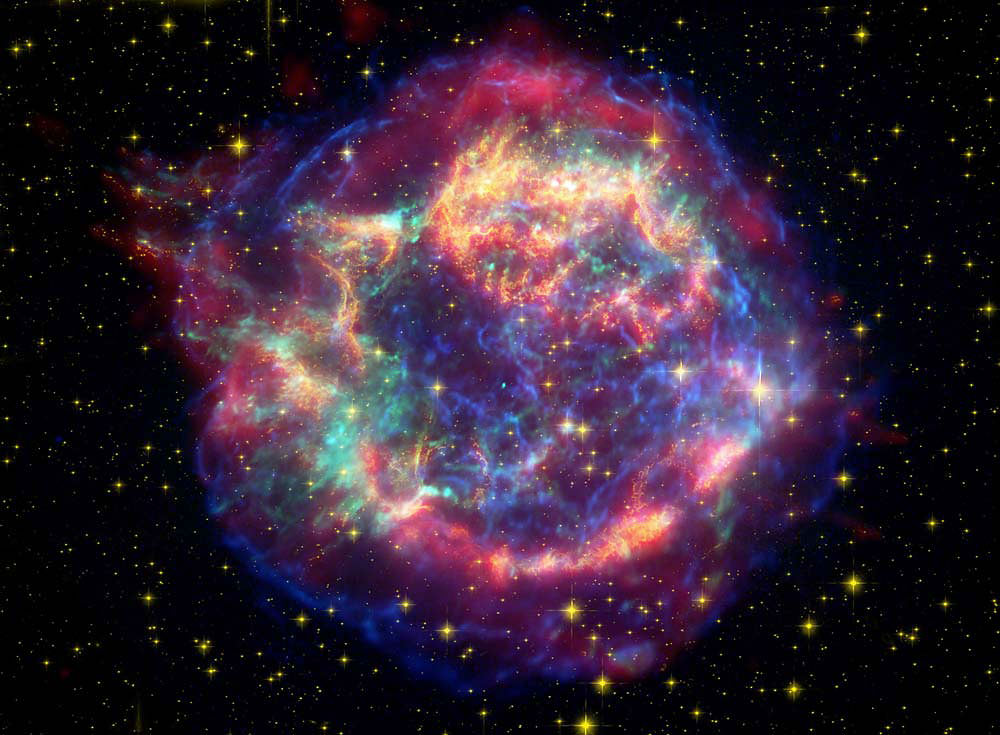
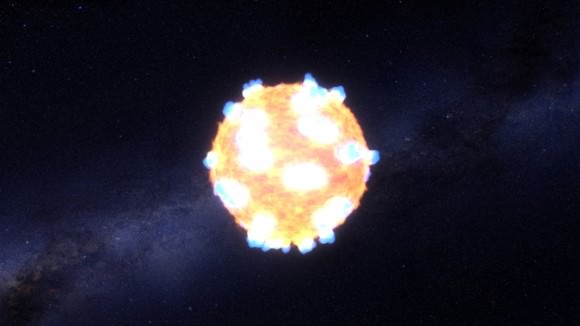
There are a lot of ways that life on Earth could come to an end: an asteroid strike, global climate catastrophe, or nuclear war are among them. But perhaps the most haunting would be death by supernova, because there’s absolutely nothing we could do about it. We’d be sitting ducks.
New research suggest that a supernova’s kill zone is bigger than we thought; about 25 light years bigger, to be exact.
Iron in the Ocean
In 2016, researchers confirmed that Earth has been hit with the effects from multiple supernovae. The presence of iron 60 in the seabed confirms it. Iron 60 is an isotope of iron produced in supernova explosions, and it was found in fossilized bacteria in sediments on the ocean floor. Those iron 60 remnants suggest that two supernovae exploded near our solar system, one between 6.5 to 8.7 million years ago, and another as recently as 2 million years ago.
Iron 60 is extremely rare here on Earth because it has a short half life of 2.6 million years. Any of the iron 60 created at the time of Earth’s formation would have decayed into something else by now. So when researchers found the iron 60 on the ocean floor, they reasoned that it must have another source, and that logical source is a supernova.
This evidence was the smoking gun for the idea that Earth has been struck by supernovae. But the questions it begs are, what effect did that supernova have on life on Earth? And how far away do we have to be from a supernova to be safe?
“…we can look for events in the history of the Earth that might be connected to them (supernova events).” – Dr. Adrian Melott, Astrophysicist, University of Kansas.
In a press release from the University of Kansas, astrophysicist Adrian Melott talked about recent research into supernovae and the effects they can have on Earth. “This research essentially proves that certain events happened in the not-too-distant past,” said Melott, a KU professor of physics and astronomy. “They make it clear approximately when they happened and how far away they were. Knowing that, we can consider what the effect may have been with definite numbers. Then we can look for events in the history of the Earth that might be connected to them.”
Earlier work suggested that a supernova kill zone is about 25-30 light years. If a supernova exploded that close to Earth, it would trigger a mass extinction. Bye-bye humanity. But new work suggests that 25 light years is an under-estimation, and that a supernova 50 light years away would be powerful enough to cause a mass extinction.
Supernovae: A Force Driving Evolution?
But extinction is just one effect a supernova could have on Earth. Supernovae can have other effects, and they might not all be negative. It’s possible that a supernovae about 2.6 million years ago even drove human evolution.
“Our local research group is working on figuring out what the effects were likely to have been,” Melott said. “We really don’t know. The events weren’t close enough to cause a big mass extinction or severe effects, but not so far away that we can ignore them either. We’re trying to decide if we should expect to have seen any effects on the ground on the Earth.”
Melott and his colleagues have written a new paper that focuses on the effects a supernova might have on Earth. In a new paper titled “A SUPERNOVA AT 50 PC: EFFECTS ON THE EARTH’S ATMOSPHERE AND BIOTA”, Melott and a team of researchers tried to shed light on Earth-supernova interactions.
The Local Bubble
There are a number of variables that come into play when trying to determine the effects of a supernova, and one of them is the idea of the Local Bubble. The Local Bubble itself is the result of one or more supernova explosion that occurred as long as 20 million years ago. The Local Bubble is a 300 light year diameter bubble of expanding gas in our arm of the Milky Way galaxy, where our Solar System currently resides. We’ve been travelling through it for the last five to ten million years. Inside this bubble, the magnetic field is weak and disordered.
Melott’s paper focused on the effects that a supernova about 2.6 million years ago would have on Earth in two instances: while both were within the Local Bubble, and while both were outside the Local Bubble.
The disrupted magnetic field inside the Local Bubble can in essence magnify the effects a supernova can have on Earth. It can increase the cosmic rays that reach Earth by a factor of a few hundred. This can increase the ionization in the Earth’s troposphere, which mean that life on Earth would be hit with more radiation.
Outside the Local Bubble, the magnetic field is more ordered, so the effect depends on the orientation of the magnetic field. The ordered magnetic field can either aim more radiation at Earth, or it could in a sense deflect it, much like our magnetosphere does now.
Focusing on the Pleistocene
Melott’s paper looks into the connection between the supernova and the global cooling that took place during the Pleistocene epoch about 2.6 million years ago. There was no mass extinction at that time, but there was an elevated extinction rate.
According to the paper, it’s possible that increased radiation from a supernova could have changed cloud formation, which would help explain a number of things that happened at the beginning of the Pleistocene. There was increased glaciation, increased species extinction, and Africa grew cooler and changed from predominantly forests to semi-arid grasslands.
Cancer and Mutation
As the paper concludes, it is difficult to know exactly what happened to Earth 2.6 million years ago when a supernova exploded in our vicinity. And it’s difficult to pinpoint an exact distance at which life on Earth would be in trouble.
But high levels of radiation from a supernova could increase the cancer rate, which could contribute to extinction. It could also increase the mutation rate, another contributor to extinction. At the highest levels modeled in this study, the radiation could even reach one kilometer deep into the ocean.
There is no real record of increased cancer in the fossil record, so this study is hampered in that sense. But overall, it’s a fascinating look at the possible interplay between cosmic events and how we and the rest of life on Earth evolved.
Sources:
- A supernova at 50 pc: Effects on the Earth’s atmosphere and biota
- Recent near-Earth supernovae probed by global deposition of interstellar radioactive 60Fe
- The locations of recent supernovae near the Sun from modelling 60Fe transport
- Proof that ancient supernovae zapped Earth sparks hunt for after effects