One of the most interesting things about space exploration is how many technologies have an impact on our ability to reach farther. New technologies that might not immediately be used in space can still eventually have a profound long-term impact. On the other hand, everyone knows some technologies will be immediately game changing. Superconductors, or materials that do not have any electrical resistance, are one of the technologies that have the potential to be game changing. However, hurdles to their practical use have limited their applicability to a relatively small sub-set of applications, like magnetic resonance imaging devices and particle accelerators. But another major hurdle to the broad use of superconductors has now been cleared – a lab at the University of Rochester (UR) has just developed one that works at almost room temperature. The big caveat is it has to be under pressure similar to that in the Earth’s core.
The superconductor the UR lab, which was run by Dr. Ranga Dias, developed is based around hydrogen. While that might not seem like an intuitive place to search for a material that doesn’t have any resistance, hydrogen compounds have long been on the superconducting roadmap. In the past, researchers have concentrated on finding “hydrides”, or combinations of hydrogen with another material, to find a mix that could be superconducting at high temperatures, albeit with extremely high pressures.
Credit: Isaac Arthur
What the UR lab did that was novel was add a third element into the mix – carbon. Carbon was mixed in with hydrogen sulfide, which was already well-established as a good “high-temperature” superconductor. As with many breakthrough scientific experiments, this mixture required them to try small tweaks to find a system that worked. In this case, each of those tweaks could prove to be rather expensive.
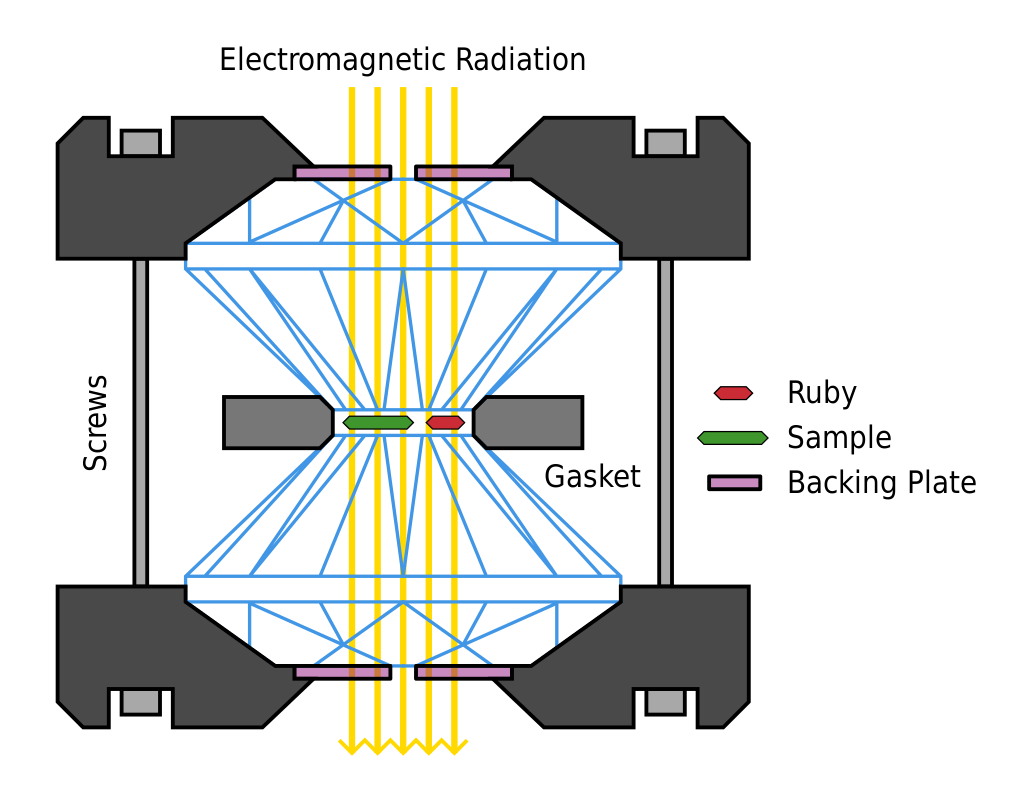
The most important component of the material mix is hydrogen. Add too little hydrogen and you won’t get a superconductive response. Add too much and the material will only superconduct at pressures that can’t possibly be reached in the lab. The key is to find a sweet spot, where the material will superconduct at pressures that are achievable using a tool known as a diamond anvil. That anvil, though it can create the highest pressures known to man, are also prone to breaking if their pressure limit is breached. Each one costs upwards of $3000, so it is likely the graduate students that performed the work had many sleepless nights calculating the literal cost of their failures.
Credit: Wikipedia user Tobias1984
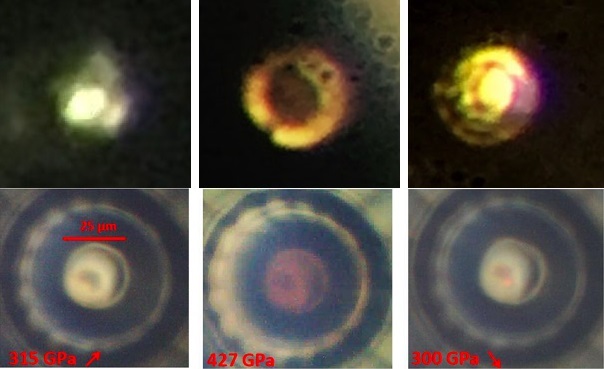
Ultimately though, they did succeed. The material that they came up with is able to superconduct at a temperature of 15 degrees Celsius, and at 267 GPa, 75% the 330 GPa present in the Earth’s core. Thankfully, that pressure does not consistently break their diamond anvils.
At such a high pressure though, that means that this specific material is unusable for any commercial applications. However, there is still much more to learn. One key characteristic of the material that has yet to be uncovered is its crystal lattice structure. Lattice structure is an important component of understanding how something superconducts. Metallic hydrogen is notoriously difficult to probe for lattice structure, as it is too small to show up on traditional techniques. This lack of understanding makes it impossible to know the exact chemical formulation of the material that was formed when the compound was put under such high pressure.
Credit: Isaac Silvera
Carbon might hold the key to eliminating the need for that pressure. Lattice structures formed with carbon are very stable compared to the lightweight bonds that hydrogen forms. If materials scientists are able to leverage that carbon structure in a way that allows the electrons to move freely at lower pressures, it could lead to a room temperature and pressure superconductor.
In the meantime, theorists and experimentalists will be in a race to develop new ideas and materials based on these findings. Almost every article on the paper has enthusiastic quotes from specialist researchers who were not part of the original work. When scientists come together to praise their colleagues’ work it’s a good sign that a true milestone has been reached. With a little more work, and after 100 years of research, we might finally be able to fully apply superconducting materials in space exploration and beyond.
Learn More:
SciShow – The First Room Temperature Superconductor!
Quanta Magazine – Room-Temperature Superconductivity Achieved for the First Time
Nature – First room-temperature superconductor excites – and baffles – scientists
Header Image Credit: J. Adam Fenster / University of Rochester


Pressure at the bottom of deep oceans is around 1GPa and it’s cold so if they can get the pressure down a bit for superconducting materials they could drastically reduce the tramsission losses of undersea cables.