Artist’s impression of an impact of two planet-sized worlds (NASA/JPL-Caltech)
Scientists have uncovered a history of violence hidden within lunar rocks, further evidence that our large, lovely Moon was born of a cataclysmic collision between worlds billions of years ago.
Using samples gathered during several Apollo missions as well as a lunar meteorite that had fallen to Earth (and using Martian meteorites as comparisons) researchers have observed a marked depletion in lunar rocks of lighter isotopes, including those of zinc — a telltale element that can be “a powerful tracer of the volatile histories of planets.”
The research utilized an advanced mass spectroscopy instrument to measure the ratios of specific isotopes present in the lunar samples. The spectrometer’s high level of precision allows for data not possible even five years ago.
Scientists have been looking for this kind of sorting by mass, called isotopic fractionation, since the Apollo missions first brought Moon rocks to Earth in the 1970s, and Frédéric Moynier, PhD, assistant professor of Earth and Planetary Sciences at Washington University in St. Louis — together with PhD student, Randal Paniello, and colleague James Day of the Scripps Institution of Oceanography — are the first to find it.
The team’s findings support a now-widely-accepted hypothesis — called the Giant Impact Theory, first suggested by PSI scientists William K. Hartmann and Donald Davis in 1975 — that the Moon was created from a collision between early Earth and a Mars-sized protoplanet about 4.5 billion years ago. The effects of the impact eventually formed the Moon and changed the evolution of our planet forever — possibly even proving crucial to the development of life on Earth.
(What would a catastrophic event like that have looked like? Probably something like this:)
Read more: What’s the Moon Made Of? Earth, Most Likely.
“This is compelling evidence of extreme volatile depletion of the moon,” said Scripps researcher James Day, a member of the team. “How do you remove all of the volatiles from a planet, or in this case a planetary body? You require some kind of wholesale melting event of the moon to provide the heat necessary to evaporate the zinc.”
In the team’s paper, published in the October 18 issue of Nature, the researchers suggest that the only way for such lunar volatiles to be absent on such a large scale would be evaporation resulting from a massive impact event.
 “When a rock is melted and then evaporated, the light isotopes enter the vapor phase faster than the heavy isotopes, so you end up with a vapor enriched in the light isotopes and a solid residue enriched in the heavier isotopes. If you lose the vapor, the residue will be enriched in the heavy isotopes compared to the starting material,” explains Moynier.
“When a rock is melted and then evaporated, the light isotopes enter the vapor phase faster than the heavy isotopes, so you end up with a vapor enriched in the light isotopes and a solid residue enriched in the heavier isotopes. If you lose the vapor, the residue will be enriched in the heavy isotopes compared to the starting material,” explains Moynier.
The fact that similar isotopic fractionation has been found in lunar samples gathered from many different locations indicates a widespread global event, and not something limited to any specific regional effect.
The next step is finding out why Earth’s crust doesn’t show an absence of similar volatiles, an investigation that may lead to clues to where Earth’s surface water came from.
“Where did all the water on Earth come from?” asked Day. “This is a very important question because if we are looking for life on other planets we have to recognize that similar conditions are probably required. So understanding how planets obtain such conditions is critical for understanding how life ultimately occurs on a planet.”
“The work also has implications for the origin of the Earth,” adds Moynier, “because the origin of the Moon was a big part of the origin of the Earth.”
Read more on the Washington University news release and at the UC San Diego news center.
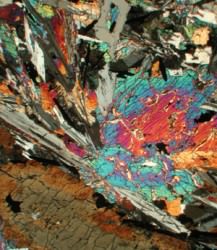
Inset image: Cross-polarized transmitted-light image of a lunar rock. Photo by James Day, Scripps/UCSD

