When we look into the night sky, we see the universe as it once was. We know that in the past the universe was once warmer and denser than it is now. When we look deep enough into the sky, we see the microwave remnant of the big bang known as the cosmic microwave background. That marks the limit of what we can see. It marks the extent of the observable universe from our vantage point.
Continue reading “Understanding the Early Universe Depends on Estimating the Lifespan of Neutrons”What Role do Radioactive Elements Play in a Planet’s Habitability?

To date, astronomers have confirmed the existence of 4,301 extrasolar planets in 3,192 star systems, with another 5,650 candidates awaiting confirmation. In the coming years, next-generation telescopes will allow astronomers to directly observe many of these exoplanets and place tighter constraints on their potential habitability. In time, this could lead to the discovery of life beyond our Solar System!
The only problem is, finding evidence of life requires that we know what to look for. According to a new study by an interdisciplinary team of scientists from the University of California Santa Cruz (UCSC), radioactive elements might play a role in planetary habitability. Future studies of rocky exoplanets, they argue, should therefore look for specific isotopes that indicate the presence of long-lived elements like thorium and uranium.
Continue reading “What Role do Radioactive Elements Play in a Planet’s Habitability?”What is Beta Radiation?
Beta radiation is radiation due to beta particles, which are electrons (or, sometimes, positrons); mostly, when you come across the words ‘beta radiation’, what is meant is what is produced by beta decay (radioactive decay which produces beta particles … either electrons or positrons).
Within a few years of Becquerel’s discovery of radioactivity (in 1896), its heterogeneous nature was discovered … and the three (then) known components given the memorable names alpha radiation, beta radiation, and gamma radiation. And, in 1900, Becquerel showed that beta radiation was composed of particles which have the same charge-to-mass ratio as electrons (which had been discovered only a few years’ earlier). The realization – by Irène and Frédéric Joliot-Curie, in 1934 – that some beta radiation is composed of positrons, rather than electrons, had to wait until positrons themselves were discovered (in 1932).
Some fun facts about beta radiation:
* beta radiation is in between alpha and gamma in terms of its penetrating power; typically it goes a meter or so in air
* like all kinds of radioactive decay, beta decay occurs because the final state of the nucleus (the one decaying) has a lower energy than the initial one (the difference is the energy of the emitted beta particle and neutrino)
* beta decay involves only the weak interaction (or force), unlike alpha and gamma decay
* the key to the specifics of beta decay is the emission of a neutrino (or antineutrino), postulated by Pauli (in 1931) and combined into a model by Fermi, in 1934 (though it wasn’t until 1956 that the neutrino was detected, and the 1960s for the existence of carriers of the weak force – the three bosons W–, W+, and Z0 – to be hypothesized).
* beta radiation has the characteristics we observe it to have because key constants in the weak interaction have the values they have (no theory in physics predicts what those values are … yet); had those values been just a teensy bit different in the early universe, we would not be here today (this is part of an idea called the anthropic principle).
Here are some of the Universe Today stories that are related to beta radiation New Insights on Magnetars, Superstrings Could Be Detectable As They Decay, and Don’t ‘Supermassive’ Me: Black Holes Regulate Their Own Mass.
Two Astronomy Cast episodes are well worth a listen, as they provide further insights into beta radiation The Strong and Weak Nuclear Forces, and Nucleosynthesis: Elements from Stars.
Beta Particles
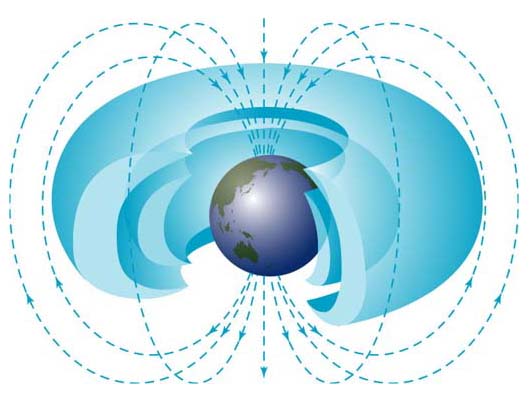
Beta particles are electrons (symbol β–), or positrons (symbol β+), emitted in beta decay (a kind of radioactivity); beta radiation in other words. Sometimes ‘beta particles’ refers to high energy electrons, irrespective of their source (e.g. the beta particles in the Van Allen radiation belts around the Earth; very few are produced by beta decay).
Of the three kinds of radioactivity (alpha, beta – both of which are particles – and gamma (which is electromagnetic radiation)), beta particles have intermediate penetrating power.
Beta particles have an important role in medicine … as diagnostic tools, to treat some diseases (notably various cancers, particularly via radionuclide therapy), in biochemical analysis, etc. For example, 18F (the fluorine-18 isotope) is used as a positron (β+) emitter in positron emission tomography (PET).
Beta particles – or rather the weak interaction which is the cause of their emission – were crucial in Big Bang Nucleosynthesis … as the early universe cooled, reactions between the protons, neutrons, electrons, and photons produced many light nuclides, but the balance between many reactions left only hydrogen, deuterium, helium-3, helium-4, and lithium-7 when the universe became too cool for any nuclear reactions to continue (of course, isolated neutrons and unstable nuclides – such as tritium – were also left, but they decayed well before the time of cosmic microwave background).
Fast forward to today … beta (β+) particles from the decay of potassium-40 is one source of internal heat for the Earth – giving us plate tectonics, its magnetic field, etc – … the decay of carbon-14 and beryllium-10 (both of which produce beta (β–) particles) provide us with tools to do radioactive (or radiometric) dating (these are two of the nuclides produced by cosmic ray spallation).
Beta Particle Radiation is a good, introductory webpage (from the University of California, Davis), and Weak Interactions explains how beta particles and the weak (nuclear) force are related (from SLAC’s Virtual Visitor Center)
Universe Today has several stories which cover the role of beta particles in astronomy; for example A Prototype Detector for Dark Matter in the Milky Way, and Fermilab Putting the Squeeze on Higgs Boson.
The Strong and Weak Nuclear Forces, and Nucleosynthesis: Elements from Stars are two Astronomy Cast episodes which will help you understand beta particles better.
What is Alpha Radiation?
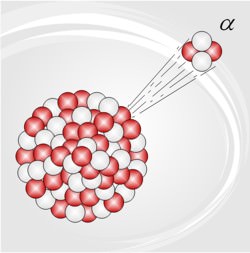
Alpha radiation is another name for the alpha particles emitted in the type of radioactive decay called alpha decay. Alpha particles are helium-4 (4He) nuclei.
Radioactivity was discovered by Becquerel, in 1896 (and one of the units of radioactivity – the becquerel – is named after him); within a few years it was discovered (Rutherford gets most of the credit, though others contributed) that there are actually three kinds of radioactivity, which were given the exciting names alpha (radiation), beta (radiation), and gamma (radiation; there are some other, rare, kinds of radioactive decay, the most important being positron, or positive beta). Rutherford (with some help) worked out that alpha radiation is actually the nuclei of helium … by allowing alpha radiation to go through the thin walls of an evacuated glass tube, and later analyzing the gas in the tube spectroscopically).
Some fun facts about alpha radiation:
* alpha radiation is the least penetrating (of alpha, beta, and gamma); typically it goes no more than a few cm in air
* like all kinds of radioactive decay, alpha decay occurs because the final state of the nucleus (the one decaying) has a lower energy than the initial one (the difference is the energy of the emitted alpha particle, both its binding energy and its kinetic energy)
* alpha decay involves both strong and electromagnetic interactions (or forces), unlike beta and gamma decay
* the key to the specifics of alpha decay is the quantum effect called tunneling; Gamow worked this out, in 1928
* only heavier nuclides can undergo alpha decay; the lightest are light isotopes of tellurium
* alpha radiation played a star role in the development of our understanding of the nature of atoms … Rutherford, in 1909, aimed a beam of alpha radiation at a piece of thin gold foil, and counted the number of particles which were deflected at each angle; from this he deduced that the atom has a very small nucleus (with all the positive charge, and nearly all its mass).
For more background on alpha radiation, check out the Jefferson Lab’s What are alpha rays? How are they produced?.
There are many ways alpha radiation can turn up in Universe Today articles; for example, in NASA May Have to Revamp Science Plans Without RTGs, alpha radiation is essential to RTGs; and in Opportunity Rover Sidelined by Charged Particle Hit, alpha radiation is what’s used to help determine the elemental composition of samples.
Nucleosynthsis: Elements from Stars and Cosmic Rays are two Astronomy Cast episodes which also cover alpha radiation.
Source: Wikipedia