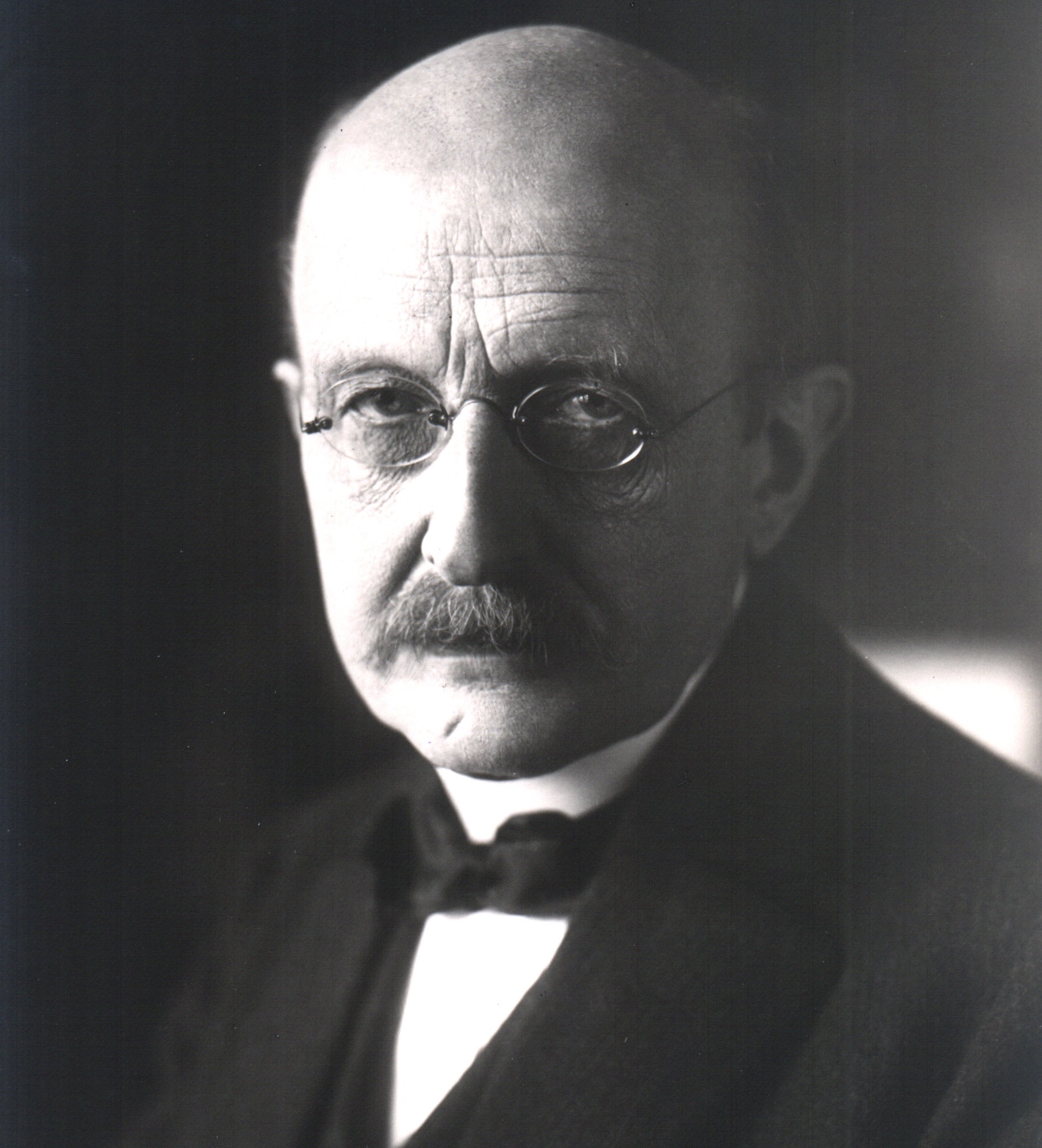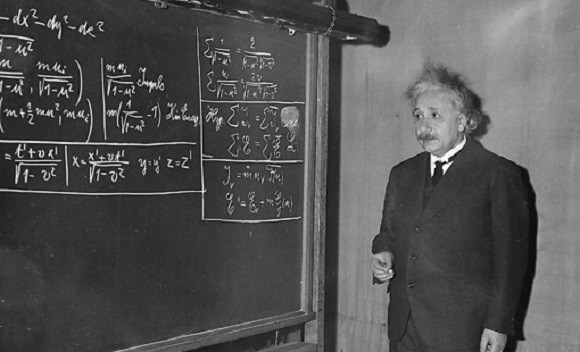Imagine if you will that your name would forever be associated with a groundbreaking scientific theory. Imagine also that your name would even be attached to a series of units, designed to performs measurements for complex equations. Now imagine that you were German who lived through two World Wars, won the Nobel Prize for physics, and outlived many of your children.
If you can do all that, then you might know what it was like to be Max Planck, the German physicist and founder of quantum theory. Much like Galileo, Newton, and Einstein, Max Planck is regarded as one of the most influential and groundbreaking scientists of his time, a man whose discoveries helped to revolutionized the field of physics. Ironic, considering that when he first embarked on his career, he was told there was nothing new to be discovered!
Early Life and Education:
Born in 1858 in Kiel, Germany, Planck was a child of intellectuals, his grandfather and great-grandfather both theology professors and his father a professor of law, and his uncle a judge. In 1867, his family moved to Munich, where Planck enrolled in the Maximilians gymnasium school. From an early age, Planck demonstrated an aptitude for mathematics, astronomy, mechanics, and music.

He graduated early, at the age of 17, and went on to study theoretical physics at the University of Munich. In 1877, he went on to Friedrich Wilhelms University in Berlin to study with physicists Hermann von Helmholtz. Helmholtz had a profound influence on Planck, who he became close friends with, and eventually Planck decided to adopt thermodynamics as his field of research.
In October 1878, he passed his qualifying exams and defended his dissertation in February of 1879 – titled “On the second law of thermodynamics”. In this work, he made the following statement, from which the modern Second Law of Thermodynamics is believed to be derived: “It is impossible to construct an engine which will work in a complete cycle, and produce no effect except the raising of a weight and cooling of a heat reservoir.”
For a time, Planck toiled away in relative anonymity because of his work with entropy (which was considered a dead field). However, he made several important discoveries in this time that would allow him to grow his reputation and gain a following. For instance, his Treatise on Thermodynamics, which was published in 1897, contained the seeds of ideas that would go on to become highly influential – i.e. black body radiation and special states of equilibrium.
With the completion of his thesis, Planck became an unpaid private lecturer at the Freidrich Wilhelms University in Munich and joined the local Physical Society. Although the academic community did not pay much attention to him, he continued his work on heat theory and came to independently discover the same theory of thermodynamics and entropy as Josiah Willard Gibbs – the American physicist who is credited with the discovery.

In 1885, the University of Kiel appointed Planck as an associate professor of theoretical physics, where he continued his studies in physical chemistry and heat systems. By 1889, he returned to Freidrich Wilhelms University in Berlin, becoming a full professor by 1892. He would remain in Berlin until his retired in January 1926, when he was succeeded by Erwin Schrodinger.
Black Body Radiation:
It was in 1894, when he was under a commission from the electric companies to develop better light bulbs, that Planck began working on the problem of black-body radiation. Physicists were already struggling to explain how the intensity of the electromagnetic radiation emitted by a perfect absorber (i.e. a black body) depended on the bodies temperature and the frequency of the radiation (i.e., the color of the light).
In time, he resolved this problem by suggesting that electromagnetic energy did not flow in a constant form but rather in discreet packets, i.e. quanta. This came to be known as the Planck postulate, which can be stated mathematically as E = hv – where E is energy, v is the frequency, and h is the Planck constant. This theory, which was not consistent with classical Newtonian mechanics, helped to trigger a revolution in science.
A deeply conservative scientists who was suspicious of the implications his theory raised, Planck indicated that he only came by his discovery reluctantly and hoped they would be proven wrong. However, the discovery of Planck’s constant would prove to have a revolutionary impact, causing scientists to break with classical physics, and leading to the creation of Planck units (length, time, mass, etc.).

Quantum Mechanics:
By the turn of the century another influential scientist by the name of Albert Einstein made several discoveries that would prove Planck’s quantum theory to be correct. The first was his theory of photons (as part of his Special Theory of Relativity) which contradicted classical physics and the theory of electrodynamics that held that light was a wave that needed a medium to propagate.
The second was Einstein’s study of the anomalous behavior of specific bodies when heated at low temperatures, another example of a phenomenon which defied classical physics. Though Planck was one of the first to recognize the significance of Einstein’s special relativity, he initially rejected the idea that light could made up of discreet quanta of matter (in this case, photons).
However, in 1911, Planck and Walther Nernst (a colleague of Planck’s) organized a conference in Brussels known as the First Solvav Conference, the subject of which was the theory of radiation and quanta. Einstein attended, and was able to convince Planck of his theories regarding specific bodies during the course of the proceedings. The two became friends and colleagues; and in 1914, Planck created a professorship for Einstein at the University of Berlin.
During the 1920s, a new theory of quantum mechanics had emerged, which was known as the “Copenhagen interpretation“. This theory, which was largely devised by German physicists Neils Bohr and Werner Heisenberg, stated that quantum mechanics can only predict probabilities; and that in general, physical systems do not have definite properties prior to being measured.

This was rejected by Planck, however, who felt that wave mechanics would soon render quantum theory unnecessary. He was joined by his colleagues Erwin Schrodinger, Max von Laue, and Einstein – all of whom wanted to save classical mechanics from the “chaos” of quantum theory. However, time would prove that both interpretations were correct (and mathematically equivalent), giving rise to theories of particle-wave duality.
World War I and World War II:
In 1914, Planck joined in the nationalistic fervor that was sweeping Germany. While not an extreme nationalist, he was a signatory of the now-infamous “Manifesto of the Ninety-Three“, a manifesto which endorsed the war and justified Germany’s participation. However, by 1915, Planck revoked parts of the Manifesto, and by 1916, he became an outspoken opponent of Germany’s annexation of other territories.
After the war, Planck was considered to be the German authority on physics, being the dean of Berlin Universit, a member of the Prussian Academy of Sciences and the German Physical Society, and president of the Kaiser Wilhelm Society (KWS, now the Max Planck Society). During the turbulent years of the 1920s, Planck used his position to raise funds for scientific research, which was often in short supply.
The Nazi seizure of power in 1933 resulted in tremendous hardship, some of which Planck personally bore witness to. This included many of his Jewish friends and colleagues being expelled from their positions and humiliated, and a large exodus of Germans scientists and academics.

Planck attempted to persevere in these years and remain out of politics, but was forced to step in to defend colleagues when threatened. In 1936, he resigned his positions as head of the KWS due to his continued support of Jewish colleagues in the Society. In 1938, he resigned as president of the Prussian Academy of Sciences due to the Nazi Party assuming control of it.
Despite these evens and the hardships brought by the war and the Allied bombing campaign, Planck and his family remained in Germany. In 1945, Planck’s son Erwin was arrested due to the attempted assassination of Hitler in the July 20th plot, for which he was executed by the Gestapo. This event caused Planck to descend into a depression from which he did not recover before his death.
Death and Legacy:
Planck died on October 4th, 1947 in Gottingen, Germany at the age of 89. He was survived by his second wife, Marga von Hoesslin, and his youngest son Hermann. Though he had been forced to resign his key positions in his later years, and spent the last few years of his life haunted by the death of his eldest son, Planck left a remarkable legacy in his wake.
In recognition for his fundamental contribution to a new branch of physics he was awarded the Nobel Prize in Physics in 1918. He was also elected to the Foreign Membership of the Royal Society in 1926, being awarded the Society’s Copley Medal in 1928. In 1909, he was invited to become the Ernest Kempton Adams Lecturer in Theoretical Physics at Columbia University in New York City.

He was also greatly respected by his colleagues and contemporaries and distinguished himself by being an integral part of the three scientific organizations that dominated the German sciences- the Prussian Academy of Sciences, the Kaiser Wilhelm Society, and the German Physical Society. The German Physical Society also created the Max Planck Medal, the first of which was awarded into 1929 to both Planck and Einstein.
The Max Planck Society was also created in the city of Gottingen in 1948 to honor his life and his achievements. This society grew in the ensuing decades, eventually absorbing the Kaiser Wilhelm Society and all its institutions. Today, the Society is recognized as being a leader in science and technology research and the foremost research organization in Europe, with 33 Nobel Prizes awarded to its scientists.
In 2009, the European Space Agency (ESA) deployed the Planck spacecraft, a space observatory which mapped the Cosmic Microwave Background (CMB) at microwave and infra-red frequencies. Between 2009 and 2013, it provided the most accurate measurements to date on the average density of ordinary matter and dark matter in the Universe, and helped resolve several questions about the early Universe and cosmic evolution.
Planck shall forever be remembered as one of the most influential scientists of the 20th century. Alongside men like Einstein, Schrodinger, Bohr, and Heisenberg (most of whom were his friends and colleagues), he helped to redefine our notions of physics and the nature of the Universe.
We have written many articles about Max Planck for Universe Today. Here’s What is Planck Time?, Planck’s First Light?, All-Sky Stunner from Planck, What is Schrodinger’s Cat?, What is the Double Slit Experiment?, and here’s a list of stories about the spacecraft that bears his name.
If you’d like more info on Max Planck, check out Max Planck’s biography from Science World and Space and Motion.
We’ve also recorded an entire episode of Astronomy Cast all about Max Planck. Listen here, Episode 218: Max Planck.
Sources: