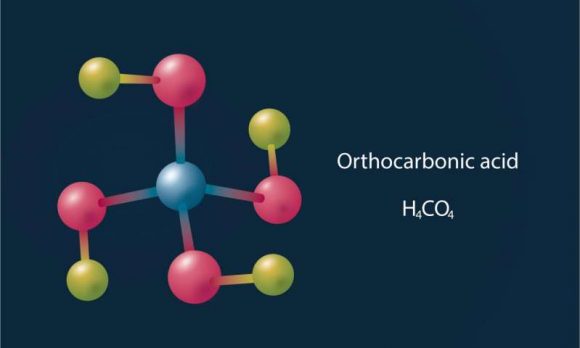
“Hitler’s acid” is a colloquial name used to refer to Orthocarbonic acid – a name which was inspired from the fact that the molecule’s appearance resembles a swastika. As chemical compounds go, it is quite exotic, and chemists are still not sure how to create it under laboratory conditions.
But it just so happens that this acid could exist in the interiors of planets like Uranus and Neptune. According to a recent study from a team of Russian chemists, the conditions inside Uranus and Neptune could be ideal for creating exotic molecular and polymeric compounds, and keeping them under stable conditions.
The study was produced by researchers from the Moscow Institute of Physics and Technology (MIPT) and the Skolkovo Institute of Science and Technology (Skoltech). Titled “Novel Stable Compounds in the C-H-O Ternary System at High Pressure”, the paper describes how the high pressure environments inside planets could create compounds that exist nowhere else in the Solar System.
Professor Artem Oganov – a professor at Skoltech and the head of MIPT’s Computational Materials Discovery Lab – is the study’s lead author. Years back, he and a team of researchers developed the worlds most powerful algorithm for predicting the formation of crystal structures and chemical compounds under extreme conditions.
Known as the Universal Structure Predictor: Evolutionary Xtallography (UPSEX), scientists have since used this algorithm to predict the existence of substances that are considered impossible in classical chemistry, but which could exist where pressures and temperatures are high enough – i.e. the interior of a planet.
With the help of Gabriele Saleh, a postdoc member of MIPT and the co-author of the paper, the two decided to use the algorithm to study how the carbon-hydrogen-oxygen system would behave under high pressure. These elements are plentiful in our Solar System, and are the basis of organic chemistry.
Until now, it has not been clear how these elements behave when subjected to extremes of temperature and pressure. What they found was that under these types of extreme conditions, which are the norm inside gas giants, these elements form some truly exotic compounds.
As Prof. Oganov explained in a MIPT press release:
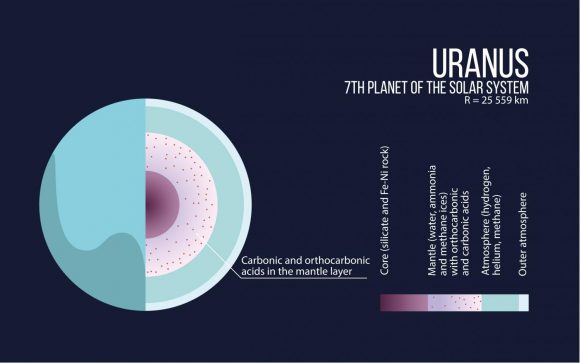
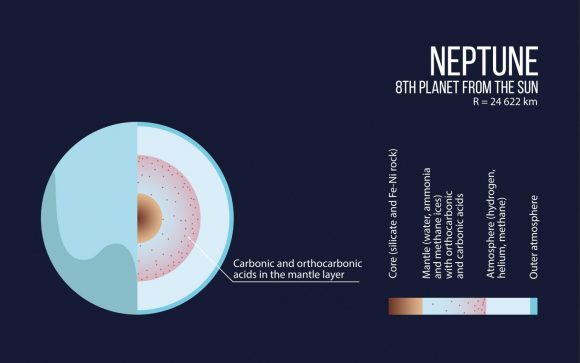
“The smaller gas giants – Uranus and Neptune – consist largely of carbon, hydrogen and oxygen. We have found that at a pressure of several million atmospheres unexpected compounds should form in their interiors. The cores of these planets may largely consist of these exotic materials.”
Under normal pressure – i.e. what we experience here on Earth (100 kPa) – any carbon, hydrogen or oxygen compounds (with the exception of methane, water and CO²) are unstable. But at pressures in the range 1 to 400 GPa (10,000 to 4 million times Earth normal), they become stable enough to form several new substances.
These include carbonic acid, orthocarbonic acid (Hitler’s acid) and other rare compounds. This was a very unusual find, considering that these chemicals are unstable under normal pressure conditions. In carbonic acid’s case, it can only remain stable when kept at very low temperatures in a vacuum.
At pressures of 314 GPa, they determined that carbonic acid (H²CO³) would react with water to form orthocarbonic acid (H4CO4). This acid is also extremely unstable, and so far, scientists have not yet been able to produce it in a laboratory environment.
This research is of considerable importance when it comes to modelling the interior of planets like Uranus and Neptune. Like all gas giants, the structure and composition of their interiors have remained the subject of speculation due to their inaccessible nature. But it could also have implications in the search for life beyond Earth.
According to Oganov and Saleh, the interiors of many moons that orbit gas giants (like Europa, Ganymede and Enceladus) also experience these types of pressure conditions. Knowing that these kinds of exotic compounds could exist in their interiors is likely to change what scientist’s think is going on under their icy surfaces.
“It was previously thought that the oceans in these satellites are in direct contact with the rocky core and a chemical reaction took place between them,” said Oganov. “Our study shows that the core should be ‘wrapped’ in a layer of crystallized carbonic acid, which means that a reaction between the core and the ocean would be impossible.”

For some time, scientists have understood that at high temperatures and pressures, the properties of matter change pretty drastically. And while here on Earth, atmospheric pressure and temperatures are quite stable (just the way we like them!), the situation in the outer Solar System is much different.
By modelling what can occur under these conditions, and knowing what chemical buildings blocks are involved, we could be able to determine with a fair degree of confidence what the interior’s of inaccessible bodies are like. This will give us something to work with when the day comes (hopefully soon) that we can investigate them directly.
Who knows? In the coming years, a mission to Europa may find that the core-mantle boundary is not a habitable environment after all. Rather than a watery environment kept warm by hydrothermal activity, it might instead by a thick layer of chemical soup.
Then again, we may find that the interaction of these chemicals with geothermal energy could produce organic life that is even more exotic!
Further Reading: MIPT, Nature Scientific Reports