While our Sun will only survive for about 5 billion more years, smaller, cooler red dwarfs can last for trillions of years. What’s the secret to their longevity?
You might say our Sun will last a long time. And sure, another 5 billion years or so of main sequence existence does sound pretty long lived. But that’s nothing compared to the least massive stars out there, the red dwarfs.
These tiny stars can have just 1/12th the mass of the Sun, but instead of living for a paltry duration, they can last for trillions of years. What’s the secret to their longevity? Is it Botox?
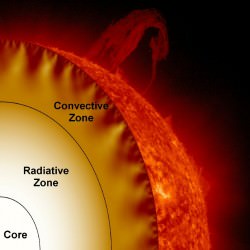
To understand why red dwarfs have such long lifespans, we’ll need to take a look at main sequence stars first, and see how they’re different. If you could peel back the Sun like a grapefruit, you’d see juicy layers inside.
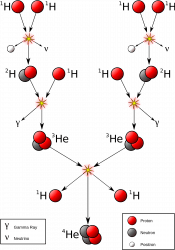
In the core, immense pressure and temperature from the mass of all that starstuff bears down and fuses atoms of hydrogen into helium, releasing gamma radiation.
Outside the core is the radiative zone, not hot enough for fusion. Instead, photons of energy generated in the core are emitted and absorbed countless times, taking a random journey to the outermost layer of the star.
And outside the radiative zone is the convective zone, where superheated globs of hot plasma float up to the surface, where they release their heat into space.
Then they cool down enough to sink back through the Sun and pick up more heat. Over time, helium builds up in the core. Eventually, this core runs out of hydrogen and it dies. Even though the core is only a fraction of the total mass of hydrogen in the Sun, there’s no mechanism to mix it in.
A red dwarf is fundamentally different than a main sequence star like the Sun. Because it has less mass, it has a core, and a convective zone, but no radiative zone. This makes all the difference.

The convective zone connects directly to the core of the red dwarf, the helium byproduct created by fusion is spread throughout the star. This convection brings fresh hydrogen into the core of the star where it can continue the fusion process.
By perfectly using all its hydrogen, the lowest mass red dwarf could sip away at its hydrogen fuel for 10 trillion years.
One of the biggest surprises in modern astronomy is just how many of these low mass red dwarf worlds have planets. And some of the most Earthlike worlds ever seen have been found around red dwarf stars. Planets with roughly the mass of Earth, orbiting within their star’s habitable zone, where liquid water could be present.
One of the biggest problems with red dwarfs is that they can be extremely variable. For example, 40% of a red dwarf’s surface could be covered with sunspots, decreasing the amount of radiation it produces, changing the size of its habitable zone.

Other red dwarfs produce powerful stellar flares that could scour a newly forming world of life. DG Canes Venaticorum recently generated a flare 10,000 times more powerful than anything ever seen from the Sun. Any life caught in the blast would have a very bad day.
Fortunately, red dwarfs only put out these powerful flares in the first billion years or so of their lives. After that, they settle down and provide a nice cozy environment for trillions of years. Long enough for life to prosper we hope.
In the distant future, some superintelligent species may figure out how to properly mix the hydrogen back into the Sun, removing the helium, if they do, they’ll add billions of years to the Sun’s life.
It seems like such a shame for the Sun to die with all that usable hydrogen sitting just a radiative zone away from fusion.
Have you got any ideas on how we could mix up the hydrogen in the Sun and remove the helium? Post your wild ideas in the comments!