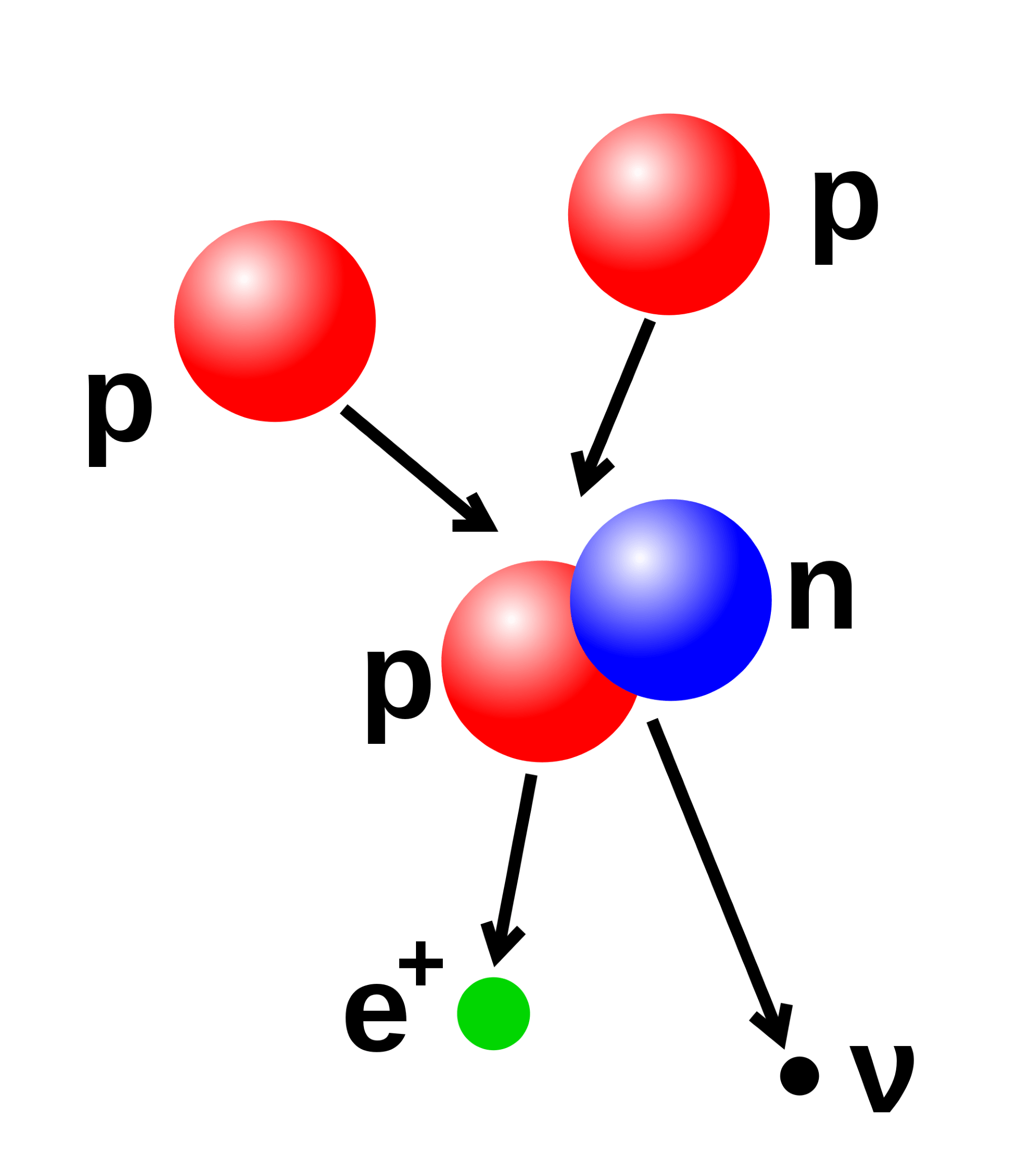
One of the biggest mysteries in the Universe is the fact there there’s matter, and not antimatter. Where did it all go?
When we look around, everything we can see is made of matter. For every type of matter from electrons, protons and quarks there is a similar type of matter known as antimatter. So why aren’t there piles of antimatter rocks, cars and chocolate bars just lying around? Why does Scotty always have a little extra kicking around in his liquor cabinet? And where do I get mine?
The primary difference between matter and antimatter is that they have opposite electric charge. Which seems pretty mundane. The negatively charged electron has an antiparticle known as the positron, which has a positive electric charge.
Anti-protons have a negative charge, and are just flat out grumpy. We’ve been able to create these particles in the lab, and have even been able to create small amounts of anti-hydrogen consisting of a positron bound to an antiproton, when examined closely there’s were shown to have a goatee and a little colorful sash or dagger.
When we create particles in accelerators such as the Large Hadron Collider, we seem to get equal amounts of matter and antimatter. This suggests that when particles were formed soon after the big bang, there should have been equal amounts of matter and antimatter.

But the universe we observe is only made of matter, and… here’s the best part… we have no idea why. Why didn’t the matter and antimatter completely annihilate each other? How come we ended up with a little more matter? This delightful mystery is known as baryon asymmetry.
We do have a few ideas, and by we, I mean some giant brained crackerjacks have come up with a few plausible options. The most popular is that somehow antimatter behaves a little differently than matter. This could cause an imbalance between matter and antimatter. After particles collided in the early universe, there would still be matter left over, hence the matter we observe.
Another idea is that the observable universe just happens to be in a region dominated by matter. Other parts of the multiverse could have observable universes dominated by antimatter. Baryon asymmetry is one of the big mysteries of modern cosmology.

There is an even crazier idea. Antimatter might have anti-gravity. In other words, an atom of anti-hydrogen would fall up instead of down. If that is the case, then matter and antimatter would repel each other, and you might have matter universes and antimatter universes that are forever separate.There have been some initial experiments to test this idea, but so far there have been no conclusive results.
What do you think? Where’s all our antimatter and how do we track it down? I’d sure love to bring some home and show my friends…
And if you like what you see, come check out our Patreon page and find out how you can get these videos early while helping us bring you more great content!