Universe Today has proudly examined the importance of studying impact craters, planetary surfaces, and exoplanets, and what they can teach scientists and the public about finding life beyond Earth. Impact craters both shape these planetary surfaces and hold the power to create or destroy life, and we learned how exoplanets are changing our views of planetary formation and evolution, including how and where we might find life in the cosmos. Here, we will discuss how these disciplines contribute to the field responsible for finding life beyond Earth, known as astrobiology. We will discuss why scientists study astrobiology, also known as astrobiologists, challenges of studying astrobiology, and how students can pursue studying astrobiology, as well. So, why is it so important to study astrobiology?
Continue reading “Astrobiology: Why study it? How to study it? What are the challenges?”An ocean floor bacteria has been found with a totally bizarre metabolism
Bacteria come in two basic forms: the kinds that use a lot of hydrogen, and the kinds that don’t. And recently researchers think they’ve found a new bacteria that appear to do both at the same time, allowing it to live in a variety of extreme environments, like the ocean floor.
Its name is Acetobacterium woodii, often shortened to A. woodii, and it seems like it’s a superhero of the small-sized world.
Continue reading “An ocean floor bacteria has been found with a totally bizarre metabolism”The Journey of Light, From the Stars to Your Eyes
This week, millions of people will turn their eyes to the skies in anticipation of the 2015 Perseid meteor shower. But what happens on less eventful nights, when we find ourselves gazing upward simply to admire the deep, dark, star-spangled sky? Far away from the glow of civilization, we humans can survey thousands of tiny pinpricks of light. But how? Where does that light come from? How does it make its way to us? And how do our brains sort all that incoming energy into such a profoundly breathtaking sight?
Our story begins lightyears away, deep in the heart of a sun-like star, where gravity’s immense inward pressure keeps temperatures high and atoms disassembled. Free protons hurtle around the core, occasionally attaining the blistering energies necessary to overcome their electromagnetic repulsion, collide, and stick together in pairs of two.
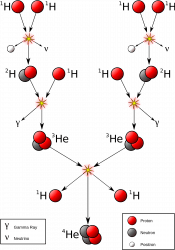
So-called diprotons are unstable and tend to disband as quickly as they arise. And if it weren’t for the subatomic antics of the weak nuclear force, this would be the end of the line: no fusion, no starlight, no us. However, on very rare occasions, a process called beta decay transforms one proton in the pair into a neutron. This new partnership forms what is known as deuterium, or heavy hydrogen, and opens the door to further nuclear fusion reactions.
Indeed, once deuterium enters the mix, particle pileups happen far more frequently. A free proton slams into deuterium, creating helium-3. Additional impacts build upon one another to forge helium-4 and heavier elements like oxygen and carbon.
Such collisions do more than just build up more massive atoms; in fact, every impact listed above releases an enormous amount of energy in the form of gamma rays. These high-energy photons streak outward, providing thermonuclear pressure that counterbalances the star’s gravity. Tens or even hundreds of thousands of years later, battered, bruised, and energetically squelched from fighting their way through a sun-sized blizzard of other particles, they emerge from the star’s surface as visible, ultraviolet, and infrared light.
Ta-da!
But this is only half the story. The light then has to stream across vast reaches of space in order to reach the Earth – a process that, provided the star of origin is in our own galaxy, can take anywhere from 4.2 years to many thousands of years! At least… from your perspective. Since photons are massless, they don’t experience any time at all! And even after eluding what, for any other massive entity in the Universe, would be downright interminable flight times, conditions still must align so that you can see even one twinkle of the light from a faraway star.
That is, it must be dark, and you must be looking up.
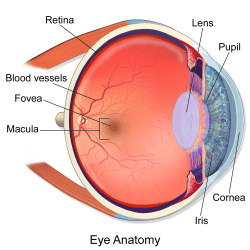
The incoming stream of photons then makes its way through your cornea and lens and onto your retina, a highly vascular layer of tissue that lines the back of the eye. There, each tiny packet of light impinges upon one of two types of photoreceptor cell: a rod, or a cone.
Most photons detected under the low-light conditions of stargazing will activate rod cells. These cells are so light-sensitive that, in dark enough conditions, they can be excited by a single photon! Rods cannot detect color, but are far more abundant than cones and are found all across the retina, including around the periphery.
The less numerous, more color-hungry cone cells are densely concentrated at the center of the retina, in a region called the fovea (this explains why dim stars that are visible in your side vision suddenly seem to disappear when you attempt to look at them straight-on). Despite their relative insensitivity, cone cells can be activated by very bright starlight, enabling you to perceive stars like Vega as blue and Betelgeuse as red.
But whether bright light or dim, every photon has the same endpoint once it reaches one of your eyes’ photoreceptors: a molecule of vitamin A, which is bound together with a specialized protein called an opsin. Vitamin A absorbs the light and triggers a signal cascade: ion channels open and charged particles rush across a membrane, generating an electrical impulse that travels up the optic nerve and into the brain. By the time this signal reaches your brain’s visual cortex, various neural pathways are already hard at work translating this complex biochemistry into what you once thought was a simple, intuitive, and poetic understanding of the heavens above…
The stars, they shine.
So the next time you go outside in the darker hours, take a moment to appreciate the great lengths it takes for just a single twinkle of light to travel from a series of nuclear reactions in the bustling center of a distant star, across the vastness of space and time, through your body’s electrochemical pathways, and into your conscious mind.
It gives every last one of those corny love songs new meaning, doesn’t it?
What Does It Mean To Be ‘Star Stuff’?

At one time or another, all science enthusiasts have heard the late Carl Sagan’s infamous words: “We are made of star stuff.” But what does that mean exactly? How could colossal balls of plasma, greedily burning away their nuclear fuel in faraway time and space, play any part in spawning the vast complexity of our Earthly world? How is it that “the nitrogen in our DNA, the calcium in our teeth, the iron in our blood, the carbon in our apple pies” could have been forged so offhandedly deep in the hearts of these massive stellar giants?
Unsurprisingly, the story is both elegant and profoundly awe-inspiring.
All stars come from humble beginnings: namely, a gigantic, rotating clump of gas and dust. Gravity drives the cloud to condense as it spins, swirling into an ever more tightly packed sphere of material. Eventually, the star-to-be becomes so dense and hot that molecules of hydrogen in its core collide and fuse into new molecules of helium. These nuclear reactions release powerful bursts of energy in the form of light. The gas shines brightly; a star is born.
The ultimate fate of our fledgling star depends on its mass. Smaller, lightweight stars burn though the hydrogen in their core more slowly than heavier stars, shining somewhat more dimly but living far longer lives. Over time, however, falling hydrogen levels at the center of the star cause fewer hydrogen fusion reactions; fewer hydrogen fusion reactions mean less energy, and therefore less outward pressure.
At a certain point, the star can no longer maintain the tension its core had been sustaining against the mass of its outer layers. Gravity tips the scale, and the outer layers begin to tumble inward on the core. But their collapse heats things up, increasing the core pressure and reversing the process once again. A new hydrogen burning shell is created just outside the core, reestablishing a buffer against the gravity of the star’s surface layers.
While the core continues conducting lower-energy helium fusion reactions, the force of the new hydrogen burning shell pushes on the star’s exterior, causing the outer layers to swell more and more. The star expands and cools into a red giant. Its outer layers will ultimately escape the pull of gravity altogether, floating off into space and leaving behind a small, dead core – a white dwarf.

Heavier stars also occasionally falter in the fight between pressure and gravity, creating new shells of atoms to fuse in the process; however, unlike smaller stars, their excess mass allows them to keep forming these layers. The result is a series of concentric spheres, each shell containing heavier elements than the one surrounding it. Hydrogen in the core gives rise to helium. Helium atoms fuse together to form carbon. Carbon combines with helium to create oxygen, which fuses into neon, then magnesium, then silicon… all the way across the periodic table to iron, where the chain ends. Such massive stars act like a furnace, driving these reactions by way of sheer available energy.
But this energy is a finite resource. Once the star’s core becomes a solid ball of iron, it can no longer fuse elements to create energy. As was the case for smaller stars, fewer energetic reactions in the core of heavyweight stars mean less outward pressure against the force of gravity. The outer layers of the star will then begin to collapse, hastening the pace of heavy element fusion and further reducing the amount of energy available to hold up those outer layers. Density increases exponentially in the shrinking core, jamming together protons and electrons so tightly that it becomes an entirely new entity: a neutron star.
At this point, the core cannot get any denser. The star’s massive outer shells – still tumbling inward and still chock-full of volatile elements – no longer have anywhere to go. They slam into the core like a speeding oil rig crashing into a brick wall, and erupt into a monstrous explosion: a supernova. The extraordinary energies generated during this blast finally allow the fusion of elements even heavier than iron, from cobalt all the way to uranium.
The energetic shock wave produced by the supernova moves out into the cosmos, disbursing heavy elements in its wake. These atoms can later be incorporated into planetary systems like our own. Given the right conditions – for instance, an appropriately stable star and a position within its Habitable Zone – these elements provide the building blocks for complex life.
Today, our everyday lives are made possible by these very atoms, forged long ago in the life and death throes of massive stars. Our ability to do anything at all – wake up from a deep sleep, enjoy a delicious meal, drive a car, write a sentence, add and subtract, solve a problem, call a friend, laugh, cry, sing, dance, run, jump, and play – is governed mostly by the behavior of tiny chains of hydrogen combined with heavier elements like carbon, nitrogen, oxygen, and phosphorus.
Other heavy elements are present in smaller quantities in the body, but are nonetheless just as vital to proper functioning. For instance, calcium, fluorine, magnesium, and silicon work alongside phosphorus to strengthen and grow our bones and teeth; ionized sodium, potassium, and chlorine play a vital role in maintaining the body’s fluid balance and electrical activity; and iron comprises the key portion of hemoglobin, the protein that equips our red blood cells with the ability to deliver the oxygen we inhale to the rest of our body.
So, the next time you are having a bad day, try this: close your eyes, take a deep breath, and contemplate the chain of events that connects your body and mind to a place billions of lightyears away, deep in the distant reaches of space and time. Recall that massive stars, many times larger than our sun, spent millions of years turning energy into matter, creating the atoms that make up every part of you, the Earth, and everyone you have ever known and loved.
We human beings are so small; and yet, the delicate dance of molecules made from this star stuff gives rise to a biology that enables us to ponder our wider Universe and how we came to exist at all. Carl Sagan himself explained it best: “Some part of our being knows this is where we came from. We long to return; and we can, because the cosmos is also within us. We’re made of star stuff. We are a way for the cosmos to know itself.”
Hunting for High Life: What Lives in Earth’s Stratosphere?
The Moon photographed through the layers of the atmosphere from the ISS in December 2003 (NASA/JSC)
What lives at the edge of space? Other than high-flying jet aircraft pilots (and the occasional daredevil skydiver) you wouldn’t expect to find many living things over 10 kilometers up — yet this is exactly where one NASA researcher is hunting for evidence of life.
 Earth’s stratosphere is not a place you’d typically think of when considering hospitable environments. High, dry, and cold, the stratosphere is the layer just above where most weather occurs, extending from about 10 km to 50 km (6 to 31 miles) above Earth’s surface. Temperatures in the lowest layers average -56 C (-68 F) with jet stream winds blowing at a steady 100 mph. Atmospheric density is less than 10% that found at sea level and oxygen is found in the form of ozone, which shields life on the surface from harmful UV radiation but leaves anything above 32 km openly exposed.
Earth’s stratosphere is not a place you’d typically think of when considering hospitable environments. High, dry, and cold, the stratosphere is the layer just above where most weather occurs, extending from about 10 km to 50 km (6 to 31 miles) above Earth’s surface. Temperatures in the lowest layers average -56 C (-68 F) with jet stream winds blowing at a steady 100 mph. Atmospheric density is less than 10% that found at sea level and oxygen is found in the form of ozone, which shields life on the surface from harmful UV radiation but leaves anything above 32 km openly exposed.
Sounds like a great place to look for life, right? Biologist David Smith of the University of Washington thinks so… he and his team have found “microbes from every major domain” traveling within upper-atmospheric winds.
Smith, principal investigator with Kennedy Space Center’s Microorganisms in the Stratosphere (MIST) project, is working to take a census of life tens of thousands of feet above the ground. Using high-altitude weather balloons and samples gathered from Mt. Bachelor Observatory in central Oregon, Smith aims to find out what kinds of microbes are found high in the atmosphere, how many there are and where they may have come from.
“Life surviving at high altitudes challenges our notion of the biosphere boundary.”
– David Smith, Biologist, University of Washington in Seattle
Although reports of microorganisms existing as high as 77 km have been around since the 1930s, Smith doubts the validity of some of the old data… the microbes could have been brought up by the research vehicles themselves.
 “Almost no controls for sterilization are reported in the papers,” he said.
“Almost no controls for sterilization are reported in the papers,” he said.
But while some researchers have suggested that the microbes could have come from outer space, Smith thinks they are terrestrial in origin. Most of the microbes discovered so far are bacterial spores — extremely hardy organisms that can form a protective shell around themselves and thus survive the low temperatures, dry conditions and high levels of radiation found in the stratosphere. Dust storms or hurricanes could presumably deliver the bacteria into the atmosphere where they form spores and are transported across the globe.
If they land in a suitable environment they have the ability to reanimate themselves, continuing to survive and multiply.
Although collecting these high-flying organisms is difficult, Smith is confident that this research will show how such basic life can travel long distances and survive even the harshest environments — not only on Earth but possibly on other worlds as well, such as the dessicated soil of Mars.
“We still have no idea where to draw the altitude boundary of the biosphere,” said Smith. This research will “address how long life can potentially remain in the stratosphere and what sorts of mutations it may inherit while aloft.”
Read more on Michael Schirber’s article for Astrobiology Magazine here, and watch David Smith’s seminar “The High Life: Airborne Microbes on the Edge of Space” held May 2012 at the University of Washington below:
Inset images – Top: layers of the atmosphere, via the Smithsonian/NMNH. Bottom: Scanning electron microscope image of atmospheric bacterial spores collected from Mt. Bachelor Observatory (NASA/KSC)
When Everything On Earth Died

[/caption]
Hey, remember that one time when 90% of all life on Earth got wiped out?
I don’t either. But it’s a good thing it happened because otherwise none of us would be here to… not remember it. Still, the end-Permian Extinction — a.k.a. the Great Dying — was very much a real crisis for life on Earth 252 million years ago. It makes the K-T extinction event of the dinosaurs look like a rather nice day by comparison, and is literally the most catastrophic event known to have ever befallen Earthly life. Luckily for us (and pretty much all of the species that have arisen since) the situation eventually sorted itself out. But how long did that take?
The Permian Extinction was a perfect storm of geological events that resulted in the disappearance of over 90% of life on Earth — both on land and in the oceans. (Or ocean, as I should say, since at that time the land mass of Earth had gathered into one enormous continent — called Pangaea — and thus there was one ocean, referred to as Panthalassa.) A combination of increased volcanism, global warming, acid rain, ocean acidification and anoxia, and the loss of shallow sea habitats (due to the single large continent) set up a series of extinctions that nearly wiped our planet’s biological slate clean.
Exactly why the event occurred and how Earth returned to a state in which live could once again thrive is still debated by scientists, but it’s now been estimated that the recovery process took about 10 million years.
(Read: Recovering From a Mass Extinction is Slow Going)
Research by Dr. Zhong-Qiang Chen from the China University of Geosciences in Wuhan, and Professor Michael Benton from the University of Bristol, UK, show that repeated setbacks in conditions on Earth continued for 5 to 6 million years after the initial wave of extinctions. It appears that every time life would begin to recover within an ecological niche, another wave of environmental calamities would break.
“Life seemed to be getting back to normal when another crisis hit and set it back again,” said Prof. Benton. “The carbon crises were repeated many times, and then finally conditions became normal again after five million years or so.”
“The causes of the killing – global warming, acid rain, ocean acidification – sound eerily familiar to us today. Perhaps we can learn something from these ancient events.”
– Michael Benton, Professor of Vertebrate Palaeontology at the University of Bristol
It wasn’t until the severity of the crises abated that life could gradually begin reclaiming and rebuilding Earth’s ecosystems. New forms of life appeared, taking advantage of open niches to grab a foothold in a new world. It was then that many of the ecosystems we see today made their start, and opened the door for the rise of Earth’s most famous prehistoric critters: the dinosaurs.
“The event had re-set evolution,” said Benton. “However, the causes of the killing – global warming, acid rain, ocean acidification – sound eerily familiar to us today. Perhaps we can learn something from these ancient events.”
The team’s research was published in the May 27 issue of Nature Geoscience. Read more on the University of Bristol’s website here.
Is Earth Alive? Scientists Seek Sulfur For An Answer
[/caption]
Researchers at the University of Maryland have discovered a way to identify and track sulfuric compounds in Earth’s marine environment, opening a path to either refute or support a decades-old hypothesis that our planet can be compared to a singular, self-regulating, living organism — a.k.a. the Gaia theory.
Proposed by scientists James Lovelock and Lynn Margulis in the 70s, the Gaia theory likens Earth to a self-supporting singular life form, similar to a cell. The theory claims that, rather than being merely a stage upon which life exists, life — in all forms — works to actively construct an Earthly environment in which it can thrive.
Although named after the Greek goddess of Earth, the Gaia theory is not so much about mythology or New Age mysticism as it is about biology, chemistry and geology — and how they all interact to make our world suitable for living things.
Once called the Gaia hypothesis, enough scientific cross-disciplinary support has since been discovered that it’s now commonly referred to as a theory.
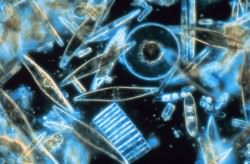
One facet of the Gaia theory is that sulfur compounds would be created by microscopic marine organisms — such as phytoplankton and algae — and these compounds could be transmitted into the air, and eventually (in some form) to the land, thus helping to support a sulfur cycle.
Sulfur is a key element in both organic and inorganic compounds. The tenth most abundant element in the Universe, sulfur is crucial to climate regulation — as well as life as we know it.
In particular, two sulfur compounds — dimethylsulfoniopropionate and its atmospherically-oxidized version, dimethylsulfide — are considered to be likely candidates for the products created by marine life. It’s these two compounds that UMD researcher Harry Oduro, along with geochemist and professor James Farquhar and marine biologist Kathryn Van Alstyne (of Western Washington University) have discovered a way to track across multiple environments, from sea to air to land, allowing scientists to trace which isotopes are coming from what sources.
“What Harry did in this research was to devise a way to isolate and measure the sulfur isotopic composition of these two sulfur compounds,” said Farquhar. “This was a very difficult measurement to do right, and his measurements revealed an unexpected variability in an isotopic signal that appears to be related to the way the sulfur is metabolized.”
The team’s research can be used to measure how the organisms are producing the compounds, under which circumstances and how they are ultimately affecting their — and our — environment in the process.
“The ability to do this could help us answer important climate questions, and ultimately better predict climate changes,” said Farquhar. “And it may even help us to better trace connections between dimethylsulfide emissions and sulfate aerosols, ultimately testing a coupling in the Gaia hypothesis.”
Whether or not Earth can be called a singular — or possibly even sentient — living organism of which all organisms are contributing members thereof may still be up for debate, but it is fairly well-accepted that life can shape and alter its own environment (and in the case of humans, often for the worse.) Research like this can help science determine just how far-reaching those alterations may be.
The study appears in this week’s Online Early Edition of the Proceedings of the National Academy of Sciences (PNAS).
Read more on the University of Maryland’s news page here.
Image credit: ESA ©2009 MPS for OSIRIS Team MPS/UPD/LAM/IAA/RSSD/INTA/UPM/DASP/IDA. Edited by J. Major.
Is This Proof of Life on Mars?
[/caption]
The Curiosity rover is currently on its way to Mars, scheduled to make a dramatic landing within Gale Crater in mid-August and begin its hunt for the geologic signatures of a watery, life-friendly past. Solid evidence that large volumes of water existed on Mars at some point would be a major step forward in the search for life on the Red Planet.
But… has it already been found? Some scientists say yes.
Researchers from universities in Los Angeles, California, Tempe, Arizona and Siena, Italy have published a paper in the International Journal of Aeronautical and Space Sciences (IJASS) citing the results of their work with data obtained by NASA’s Viking mission.
The twin Viking 1 and 2 landers launched in August and September of 1975 and successfully landed on Mars in July and September of the following year. Their principal mission was to search for life, which they did by digging into the ruddy Martian soil looking for signs of respiration — a signal of biological activity.

The results, although promising, were inconclusive.
Now, 35 years later, one team of researchers claims that the Viking landers did indeed detect life, and the data’s been there all along.
“Active soils exhibited rapid, substantial gas release,” the team’s report states. “The gas was probably CO2 and, possibly, other radiocarbon-containing gases.”
By applying mathematical complexities to the Viking data for deeper analysis, the researchers found that the Martian samples behaved differently than a non-biological control group.
“Control responses that exhibit relatively low initial order rapidly devolve into near-random noise, while the active experiments exhibit higher initial order which decays only slowly,” the paper states. “This suggests a robust biological response.”
While some critics of the findings claim that such a process of identifying life has not yet been perfected — not even here on Earth — the results are certainly intriguing… enough to bolster support for further investigation into Viking data and perhaps re-evaluate the historic mission’s “inconclusive” findings.
The team’s paper can be found here.
Image credits: NASA/JPL-Caltech. Also, read more on Irene Klotz’s article on Discovery News.
Replication of Arsenic Life Experiment Not Successful So Far

[/caption]
One of the most vocal and ardent critics of the so-called ‘arsenic life’ experiment which was published in December 2010 was biologist Rosie Redfield from the University of British Columbia in Vancouver. The science paper by NASA astrobiologist Felisa Wolfe-Simon and her team reported that a type of bacteria in Mono Lake in California can live and grow almost entirely on arsenic, a poison, and incorporates it into its DNA. Redfield called the paper “lots of flim-flam, but very little reliable information.” Her opinion was quickly seconded by many other biologists/bloggers.
Redfield has been working on replicating the experiment done by Wolfe-Simon, and doing in her work in front of the world, so to speak. She is detailing her work in an open lab notebook on her blog. So far, she reports that her results contradict Wolfe-Simon et al.’s observations.
To date, Redfield is finding that the bacteria, called GFAJ-1, is not living and growing in arsenic, but dying. Redfield says her work refutes that cells from the GFAJ-1 could use arsenic for growth in place of phosphorus, and when arsenic was added to the low-phosphorus medium in which the bacteria was living, the bacteria was killed. Additionally, in other test viles, the growth properties Redfield is finding for GFAJ-1 don’t match those reported by Wolfe-Simon and her team, which claimed that the bacteria could not grow on a low concentration of phosphorus, and that the bacteria could grow on arsenic in the absence of phosphorus.

Redfield’s two major early criticisms of the original paper were that the authors had not ruled out the possibility that the bacteria were feeding on phosphorus contaminating their growth medium; and that the bacterial DNA was not properly purified, so that the arsenic detected might not actually have been in DNA.
An article in Nature reports that other researchers also working on replicating the experiment with GFAJ-1 laud Redfield’s efforts, but say it is too early to conclude that she has debunked the original work.
Additionally, one problem is that Redfield she did not replicate the experiment exactly, as she had to add one nutrient not used by the authors of the original arsenic life paper in order for the bacteria to grow.
This is not the first time scientists have written open notebooks during the replication of controversial findings, but it might be one of the more notable, given the amount of media attention the arsenic life paper received.
Redfield is also hoping that her work will highlight the benefits of open notebook-type research.
You can read Redfield’s blog about her work at this link.
Sources: Nature, Redfield’s blog.